Summary information and primary citation
- PDB-id
- 3pkm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (3.103 Å)
- Summary
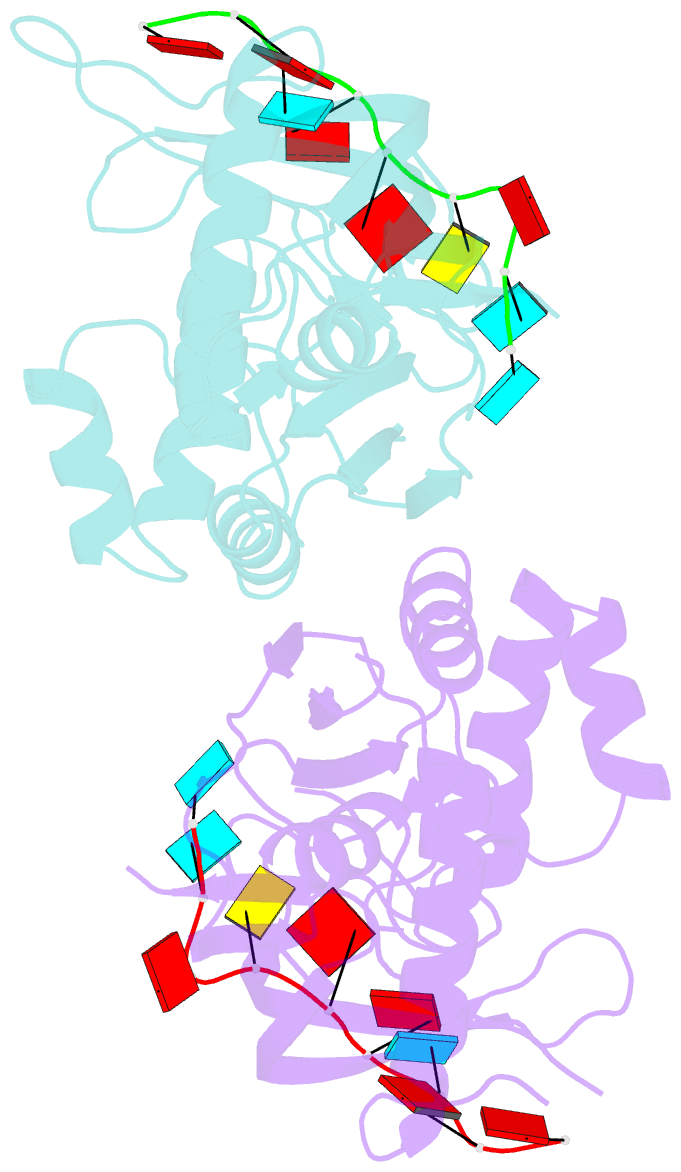
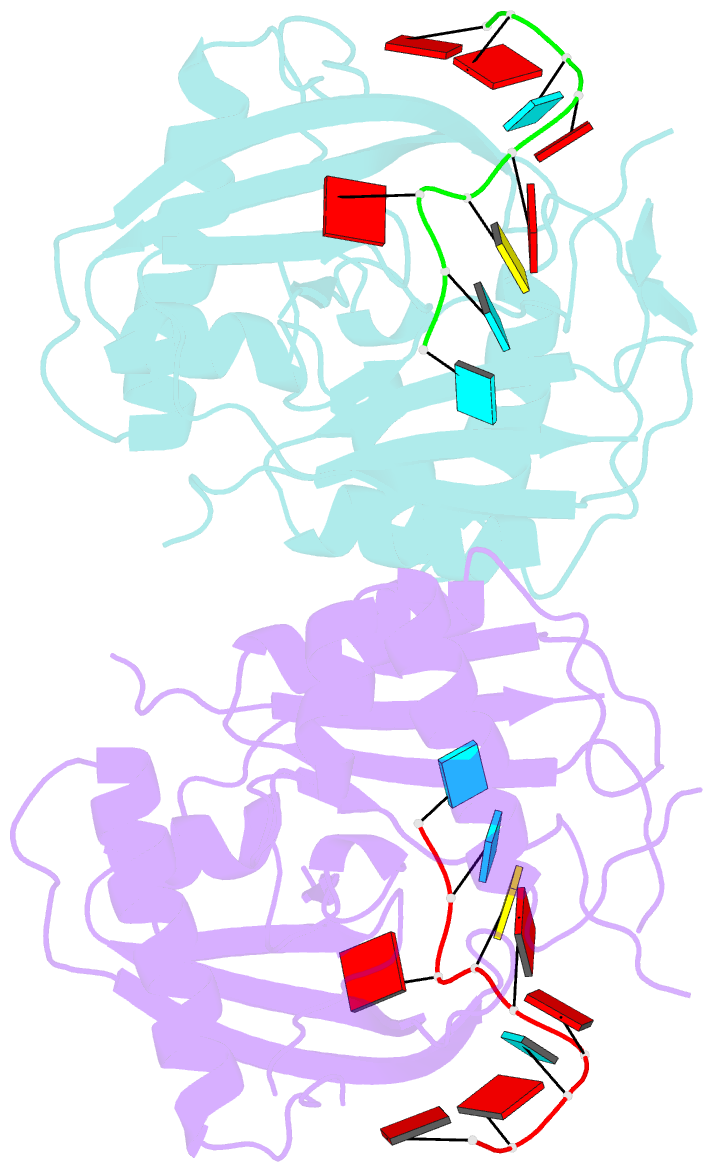
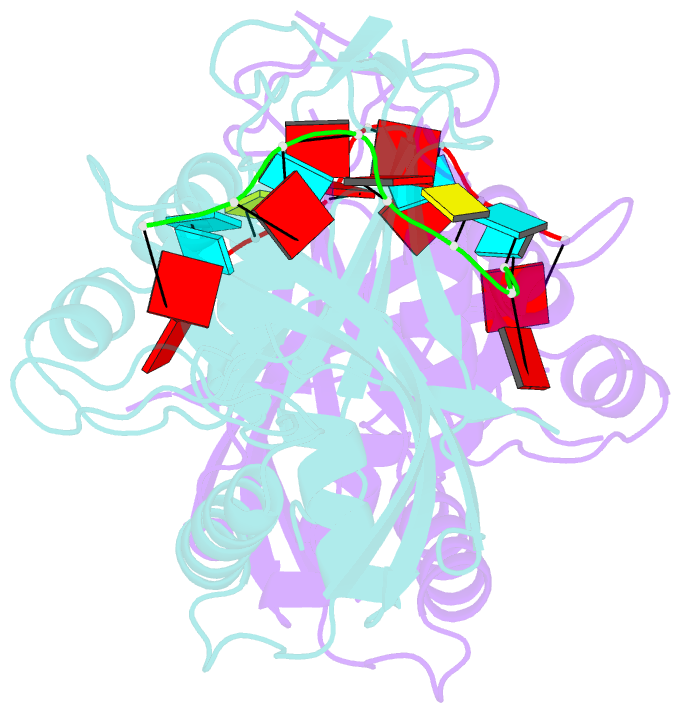
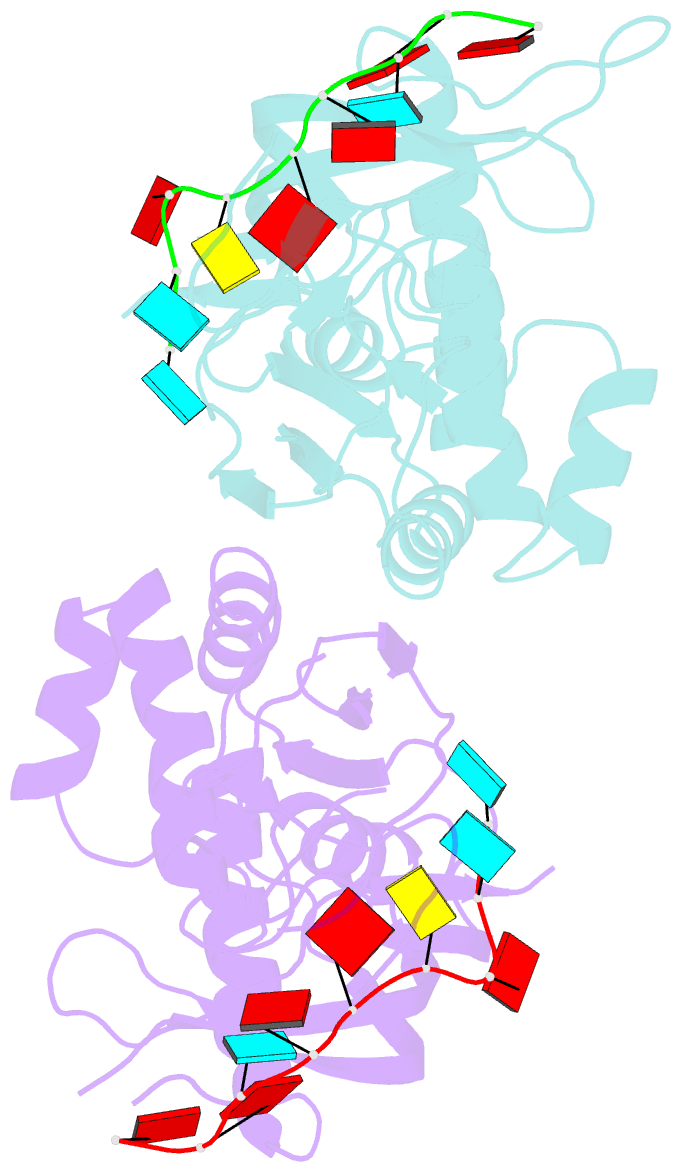
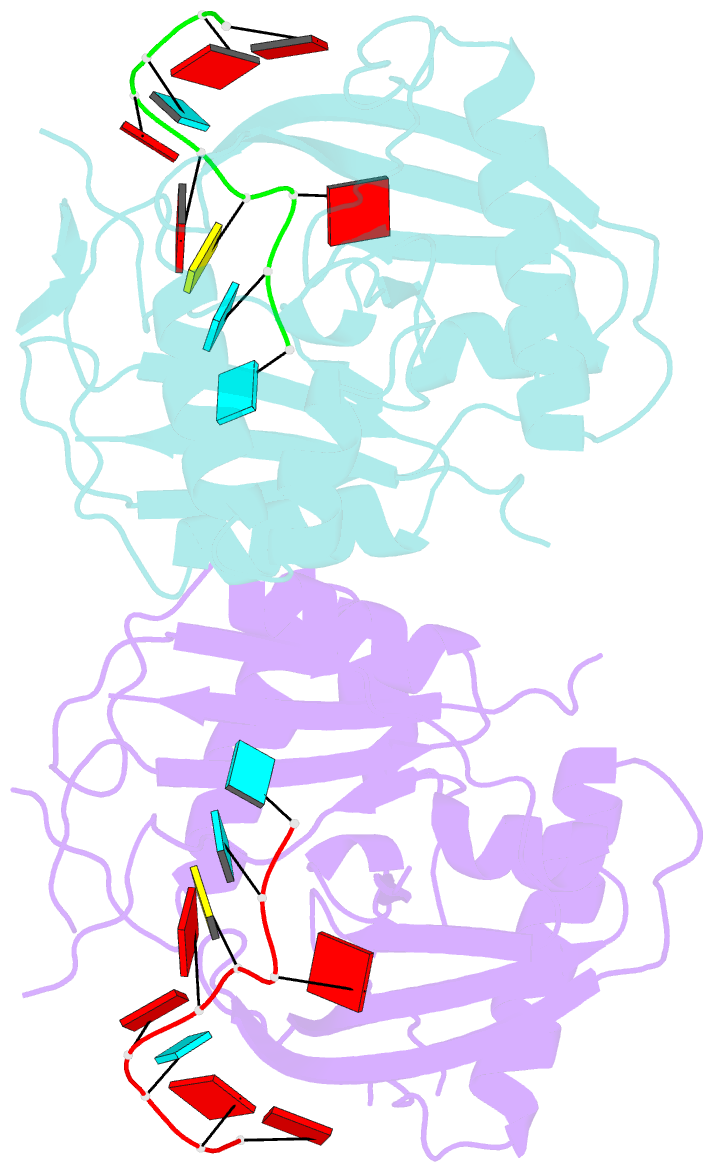
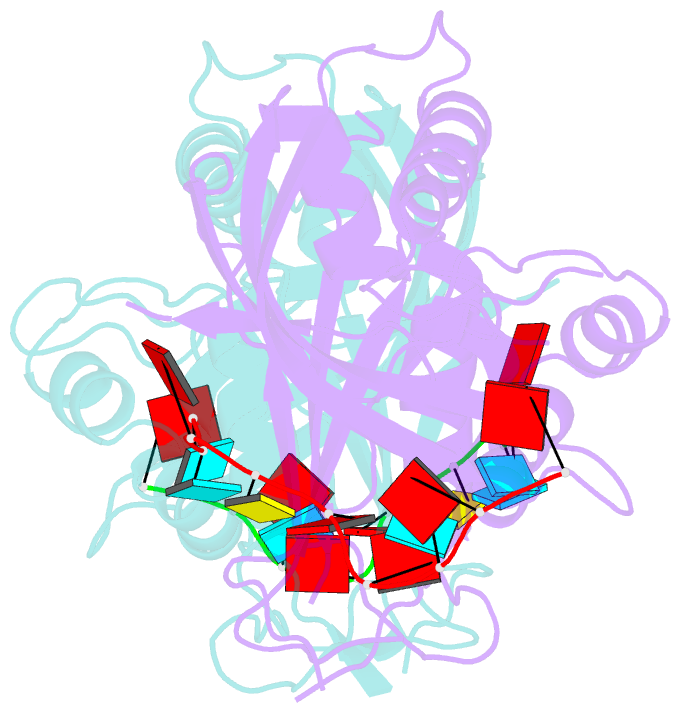
- Crystal structure of cas6 with its substrate RNA
- Reference
- Wang R, Preamplume G, Terns MP, Terns RM, Li H (2011): "Interaction of the Cas6 Riboendonuclease with CRISPR RNAs: Recognition and Cleavage." Structure, 19, 257-264. doi: 10.1016/j.str.2010.11.014.
- Abstract
- The CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats) found in prokaryotic genomes confer small RNA-mediated protection against viruses and other invaders. CRISPR loci contain iterations of a short repeat sequence alternating with small segments of varying invader-derived sequences. Distinct families of CRISPR-associated Cas proteins function to cleave within the repeat sequence of CRISPR transcripts and produce the individual invader-targeting crRNAs. Here, we report the crystal structure of Pyrococcus furiosus Cas6 bound with a repeat RNA at 3.2 Å resolution. In contrast to other Cas families of endonucleases, Cas6 clasps nucleotides 2-9 of the repeat RNA using its two ferredoxin-like domains, and the enzyme-anchored 5' end tethers the distal cleavage site of the RNA between nucleotides 22 and 23 to the predicted enzyme active site on the opposite side of the ferrodoxin-like domains. Our findings suggest a wrap-around mechanism for CRISPR RNA recognition and cleavage by Cas6 and related processing endonucleases.