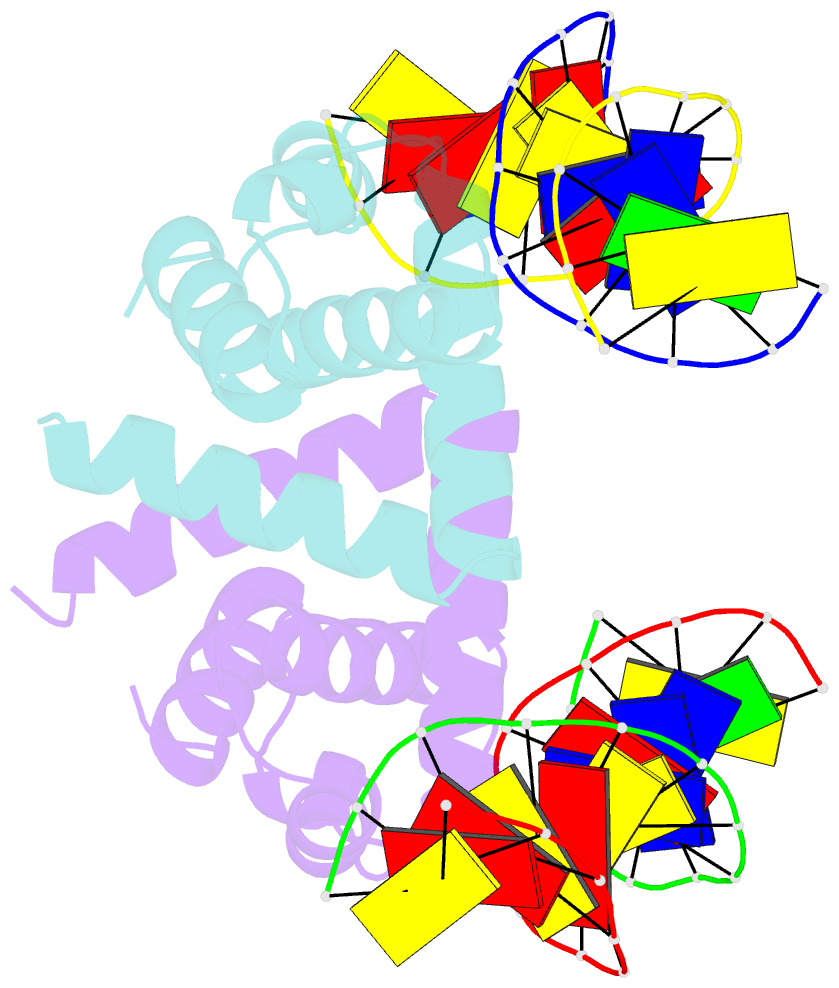
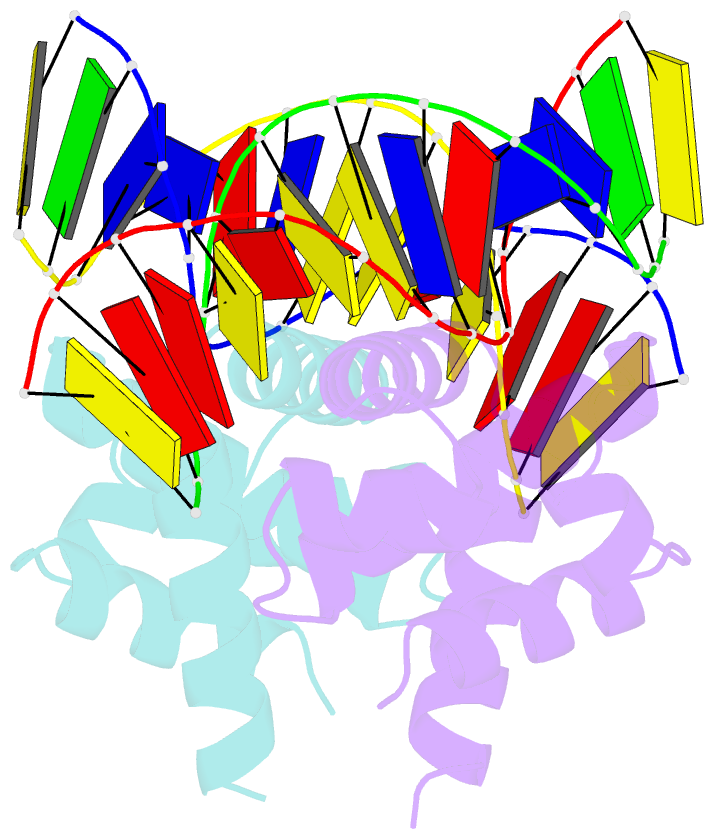
Summary information and primary citation
- PDB-id
- 3pvp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.3 Å)
- Summary
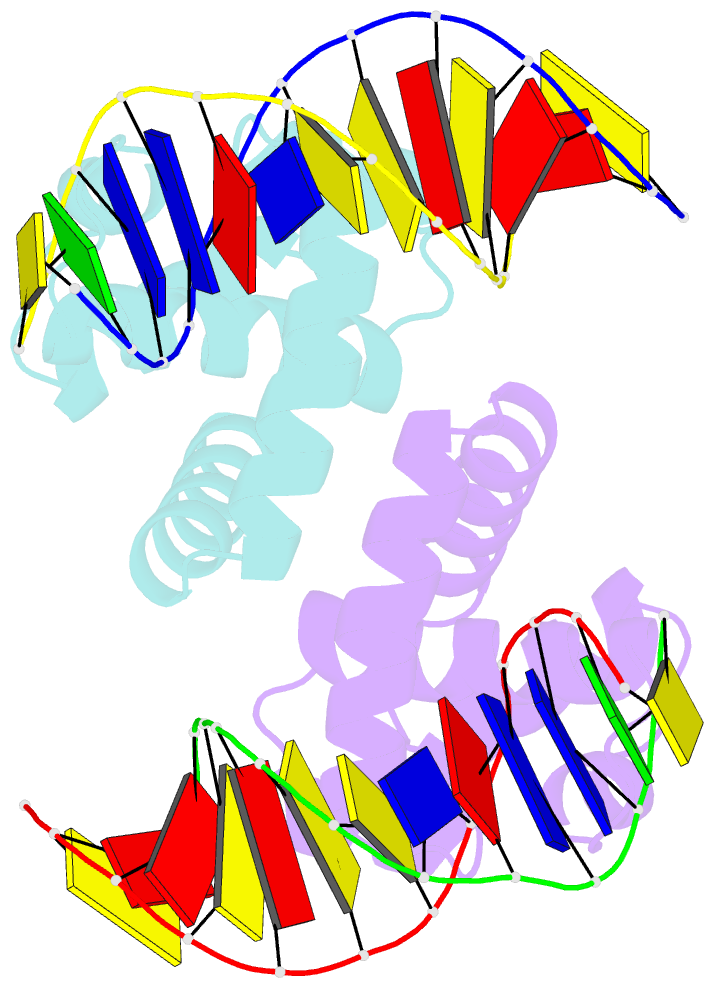
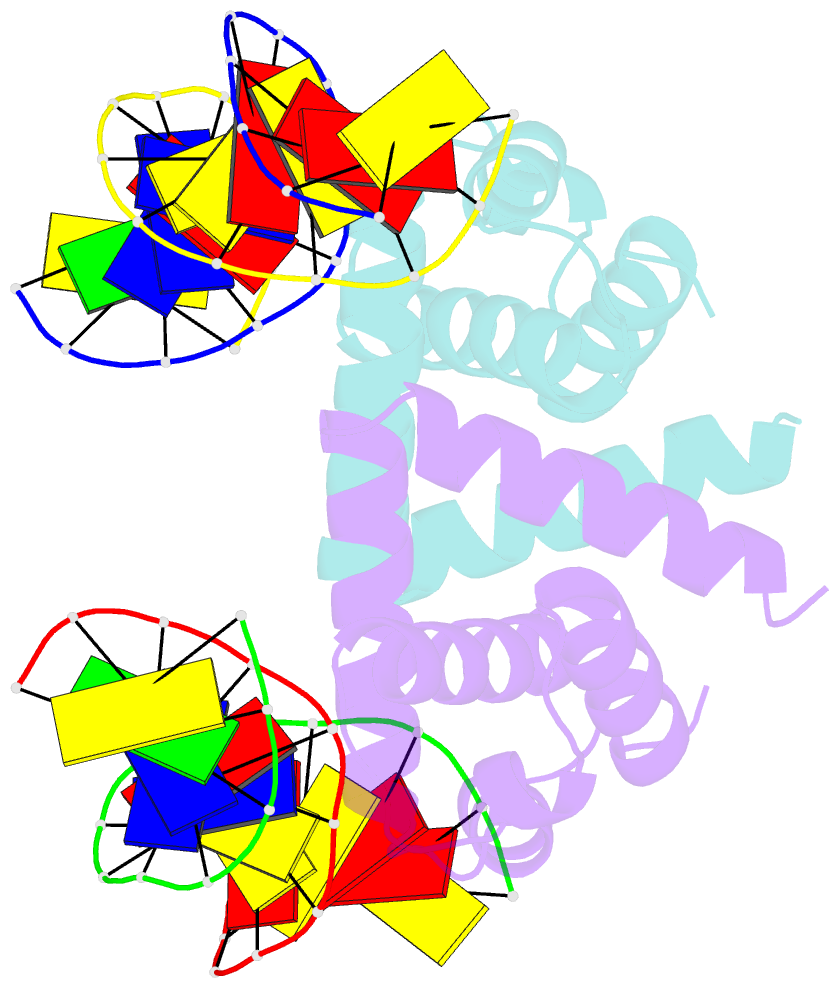
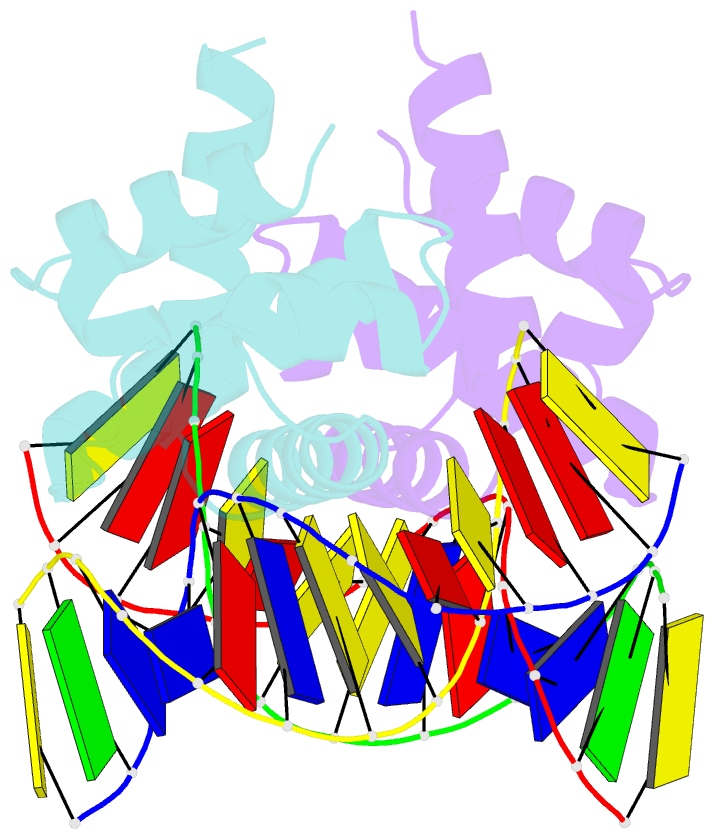
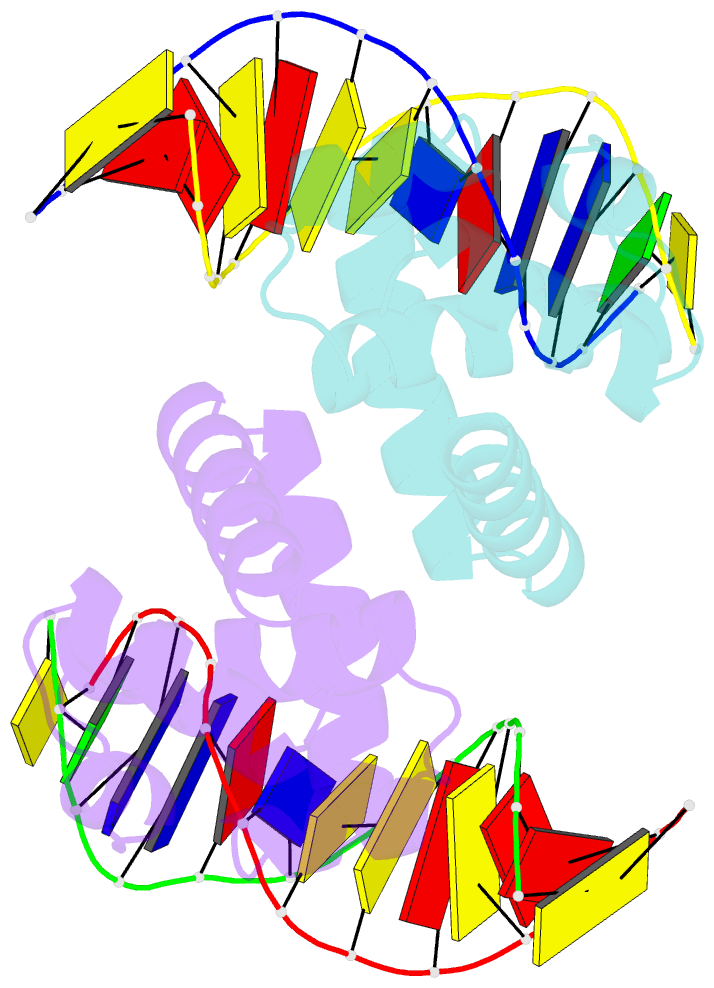
- Structure of mycobacterium tuberculosis dnaa-dbd in complex with box2 DNA
- Reference
- Tsodikov OV, Biswas T (2011): "Structural and Thermodynamic Signatures of DNA Recognition by Mycobacterium tuberculosis DnaA." J.Mol.Biol., 410, 461-476. doi: 10.1016/j.jmb.2011.05.007.
- Abstract
- An essential protein, DnaA, binds to 9-bp DNA sites within the origin of replication oriC. These binding events are prerequisite to forming an enigmatic nucleoprotein scaffold that initiates replication. The number, sequences, positions, and orientations of these short DNA sites, or DnaA boxes, within the oriCs of different bacteria vary considerably. To investigate features of DnaA boxes that are important for binding Mycobacterium tuberculosis DnaA (MtDnaA), we have determined the crystal structures of the DNA binding domain (DBD) of MtDnaA bound to a cognate MtDnaA-box (at 2.0 Å resolution) and to a consensus Escherichia coli DnaA-box (at 2.3 Å). These structures, complemented by calorimetric equilibrium binding studies of MtDnaA DBD in a series of DnaA-box variants, reveal the main determinants of DNA recognition and establish the [T/C][T/A][G/A]TCCACA sequence as a high-affinity MtDnaA-box. Bioinformatic and calorimetric analyses indicate that DnaA-box sequences in mycobacterial oriCs generally differ from the optimal binding sequence. This sequence variation occurs commonly at the first 2 bp, making an in vivo mycobacterial DnaA-box effectively a 7-mer and not a 9-mer. We demonstrate that the decrease in the affinity of these MtDnaA-box variants for MtDnaA DBD relative to that of the highest-affinity box TTGTCCACA is less than 10-fold. The understanding of DnaA-box recognition by MtDnaA and E. coli DnaA enables one to map DnaA-box sequences in the genomes of M. tuberculosis and other eubacteria.