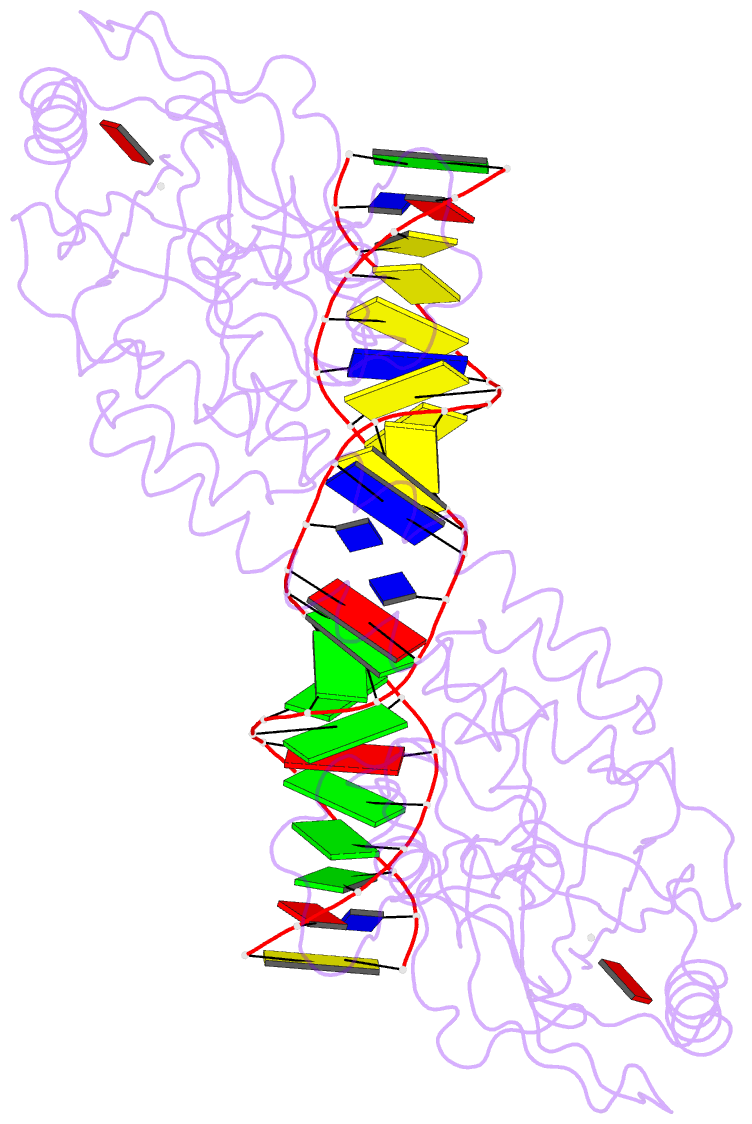
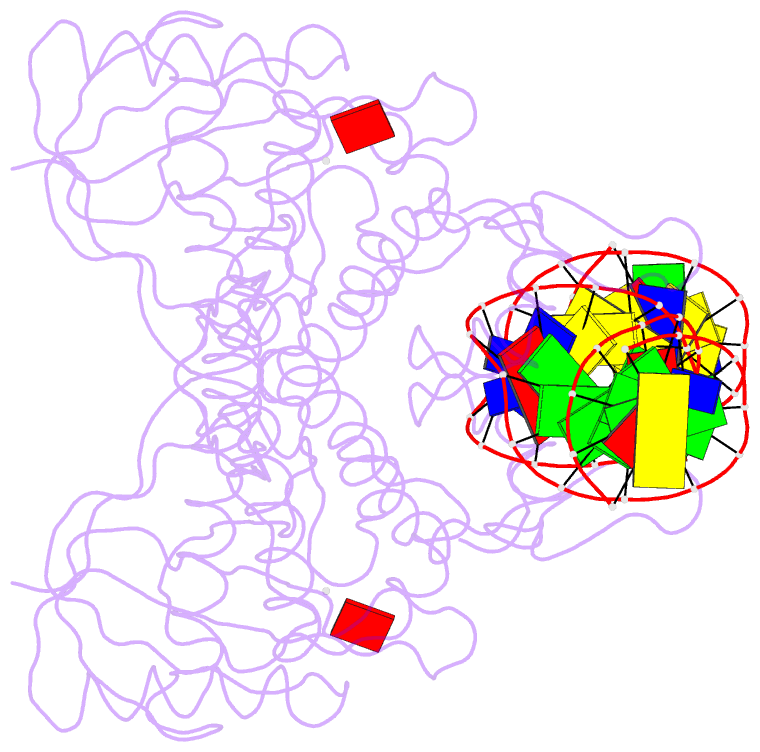
Summary information and primary citation
- PDB-id
- 3q3d; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription regulator-DNA-antibiotic
- Method
- X-ray (2.79 Å)
- Summary
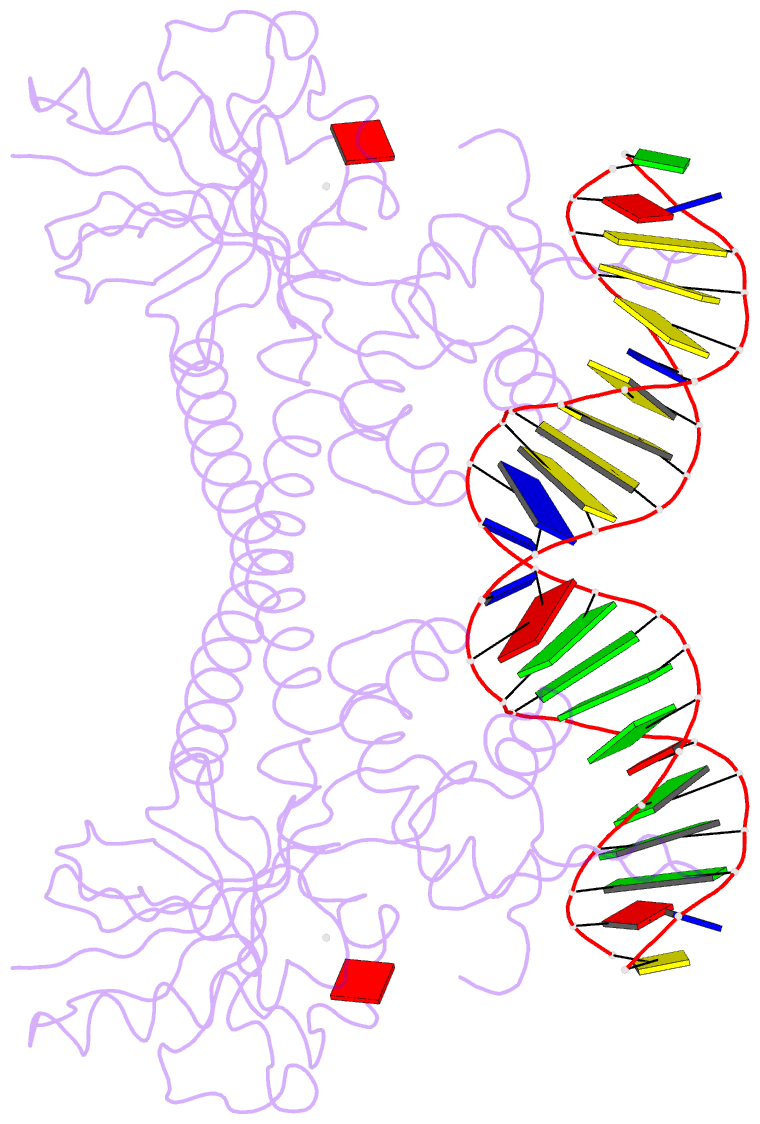
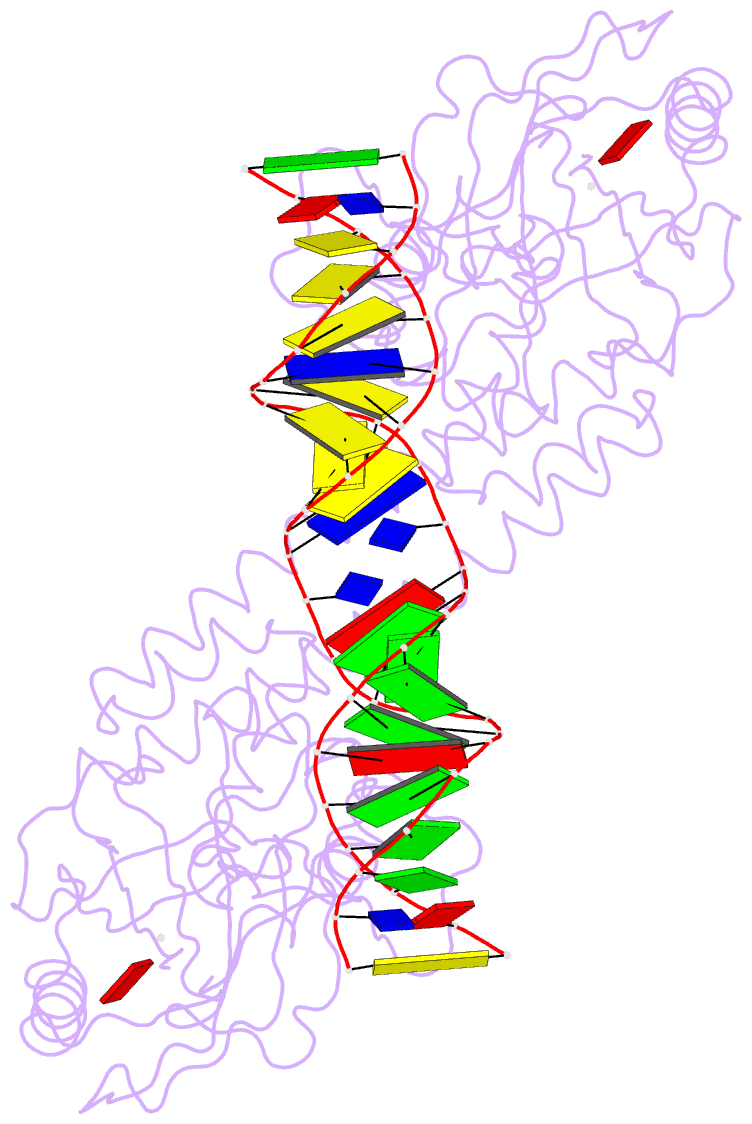
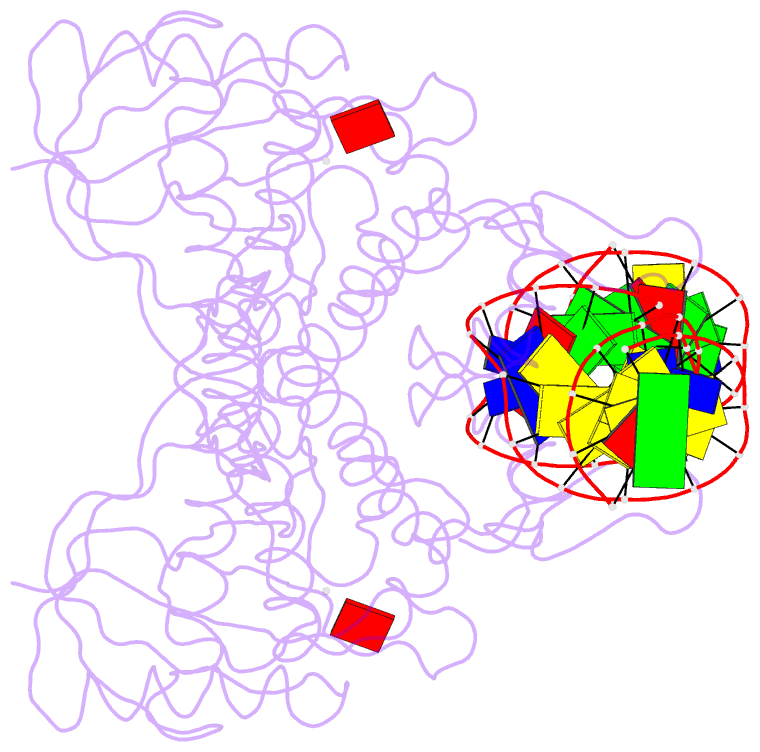
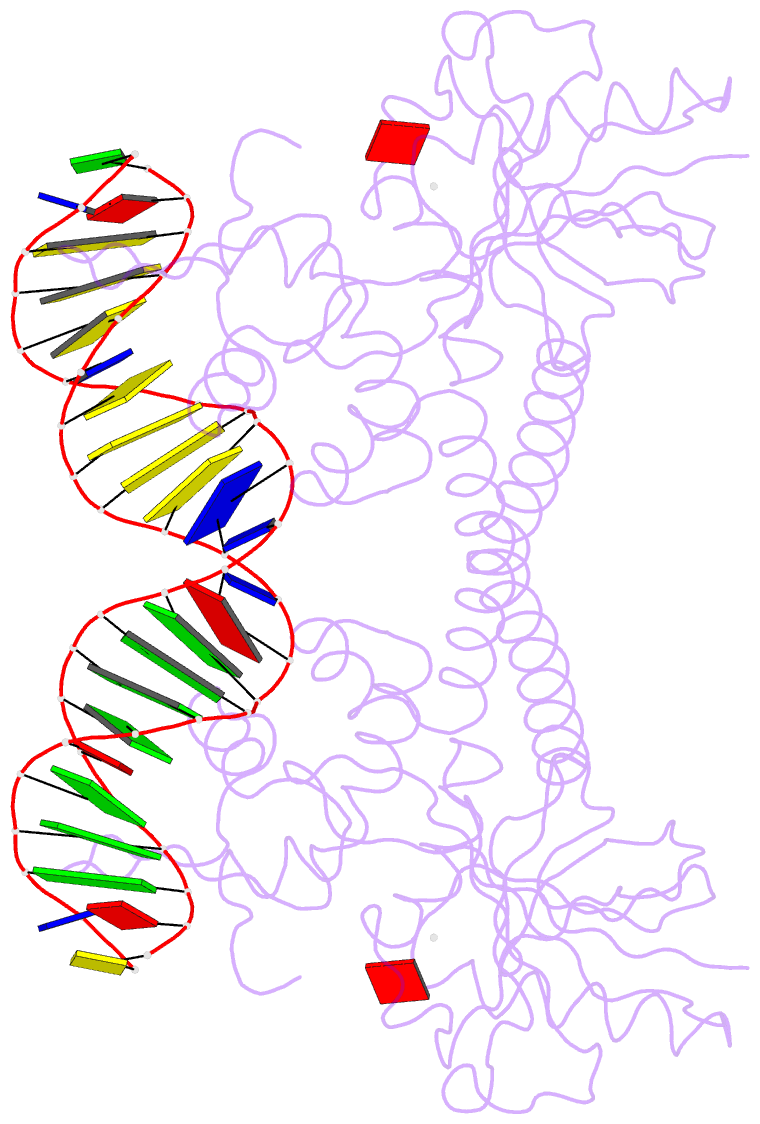
- Crystal structure of bmrr bound to puromycin
- Reference
- Bachas S, Eginton C, Gunio D, Wade H (2011): "Structural contributions to multidrug recognition in the multidrug resistance (MDR) gene regulator, BmrR." Proc.Natl.Acad.Sci.USA, 108, 11046-11051. doi: 10.1073/pnas.1104850108.
- Abstract
- Current views of multidrug (MD) recognition focus on large drug-binding cavities with flexible elements. However, MD recognition in BmrR is supported by a small, rigid drug-binding pocket. Here, a detailed description of MD binding by the noncanonical BmrR protein is offered through the combined use of X-ray and solution studies. Low shape complementarity, suboptimal packing, and efficient burial of a diverse set of ligands is facilitated by an aromatic docking platform formed by a set of conformationally fixed aromatic residues, hydrophobic pincer pair that locks the different drug structures on the adaptable platform surface, and a trio of acidic residues that enables cation selectivity without much regard to ligand structure. Within the binding pocket is a set of BmrR-derived H-bonding donor and acceptors that solvate a wide range of ligand polar substituent arrangements in a manner analogous to aqueous solvent. Energetic analyses of MD binding by BmrR are consistent with structural data. A common binding orientation for the different BmrR ligands is in line with promiscuous allosteric regulation.