Summary information and primary citation
- PDB-id
- 3q5f; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.96 Å)
- Summary
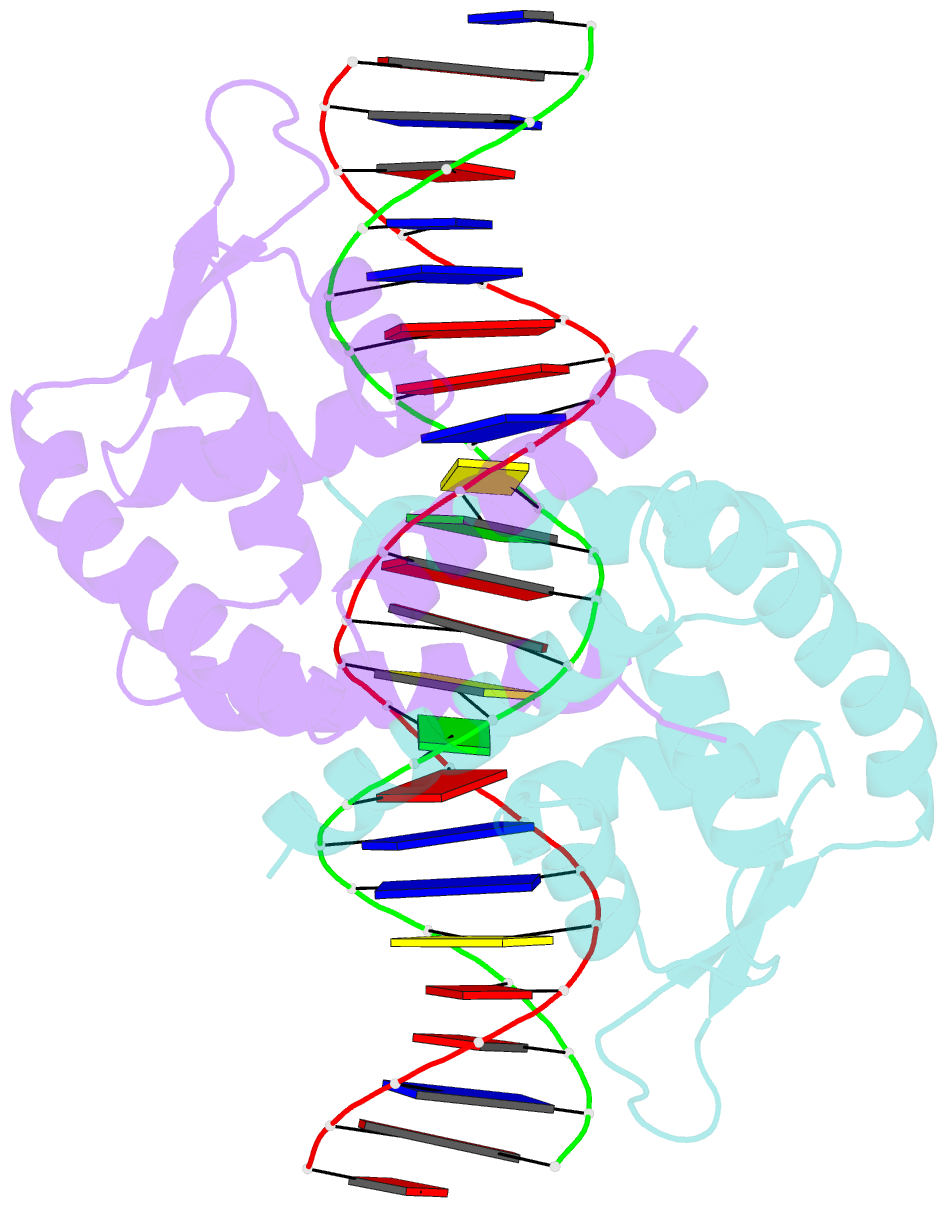
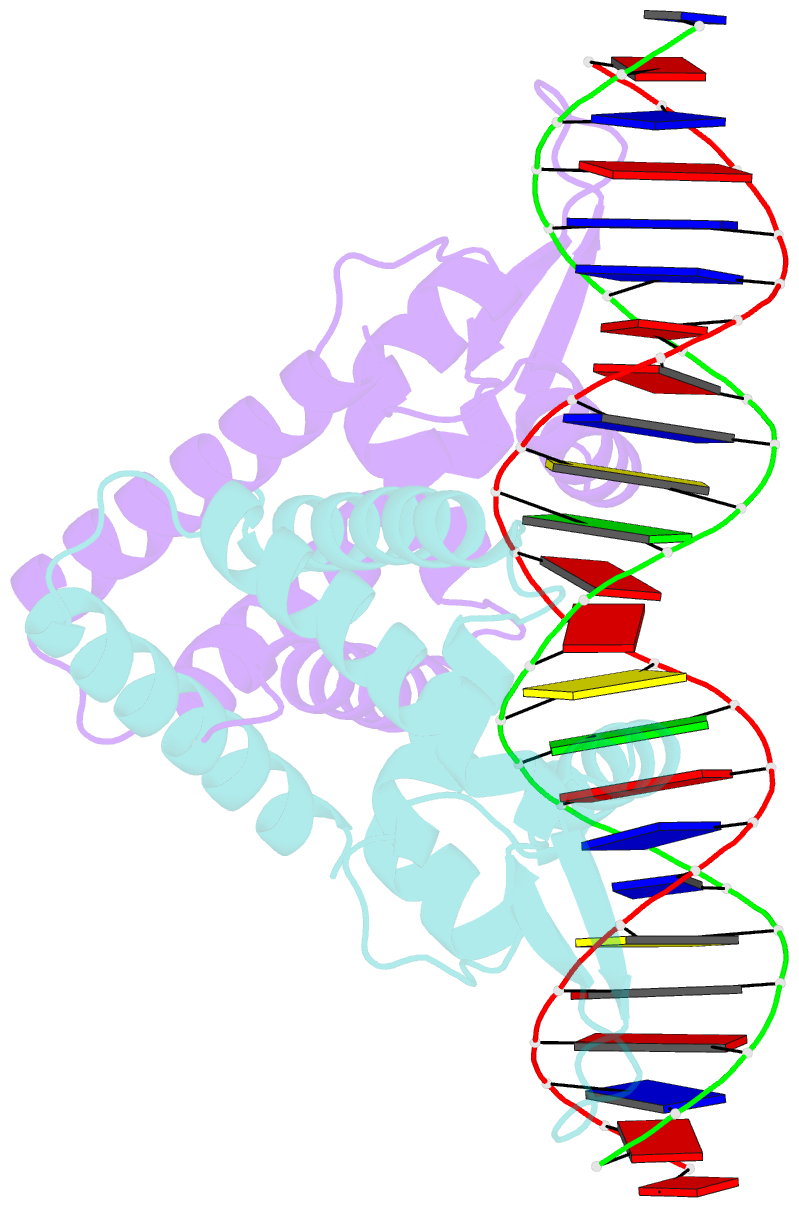
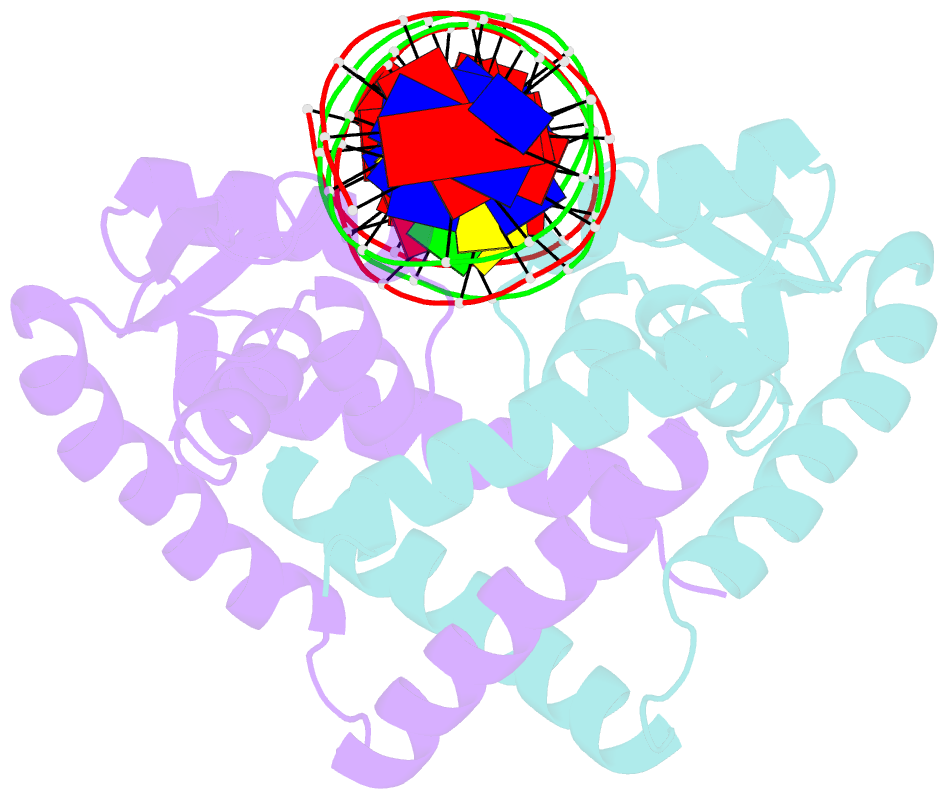
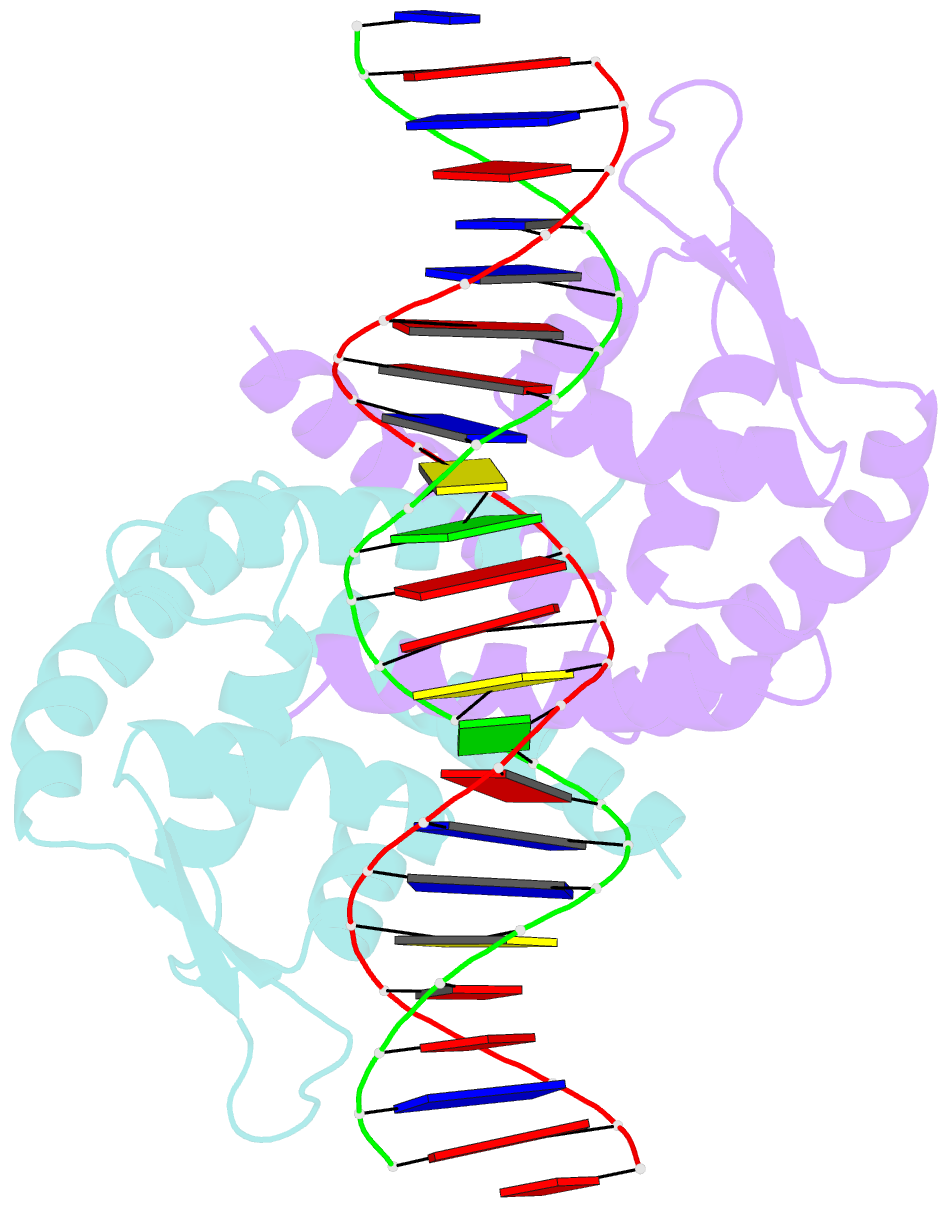
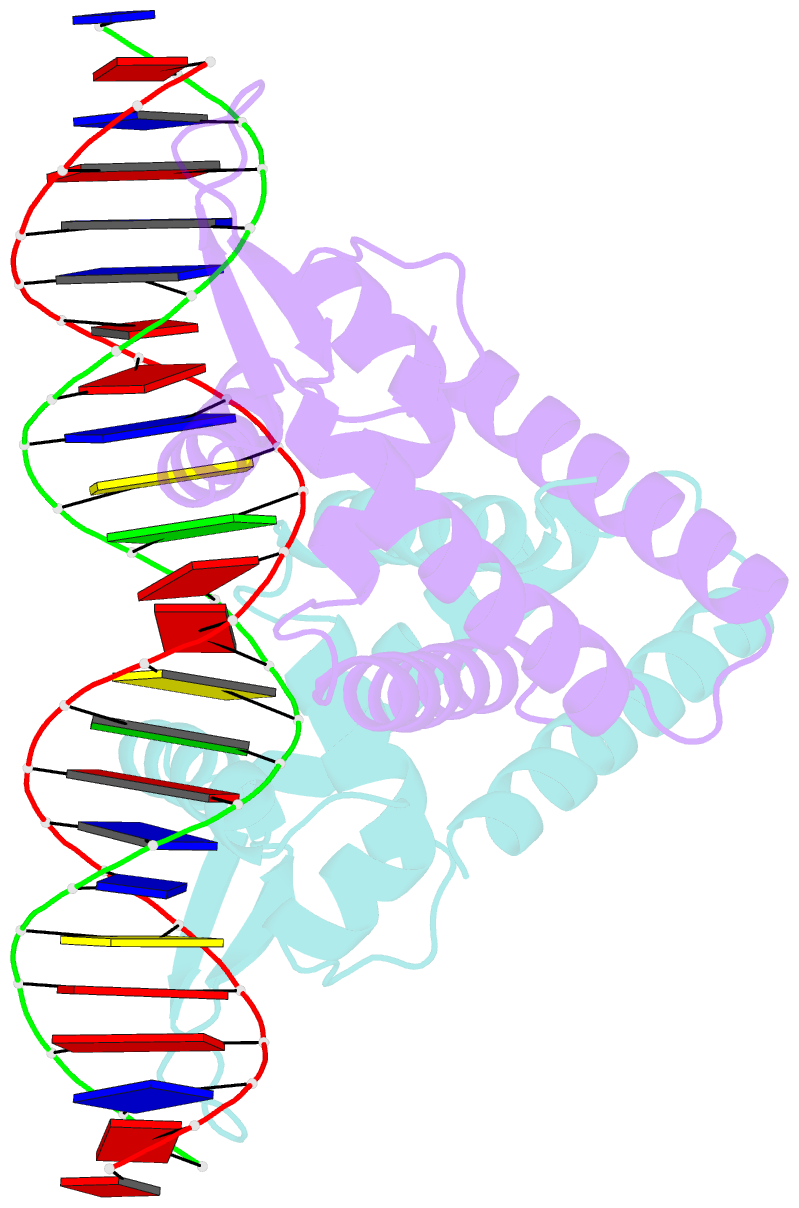
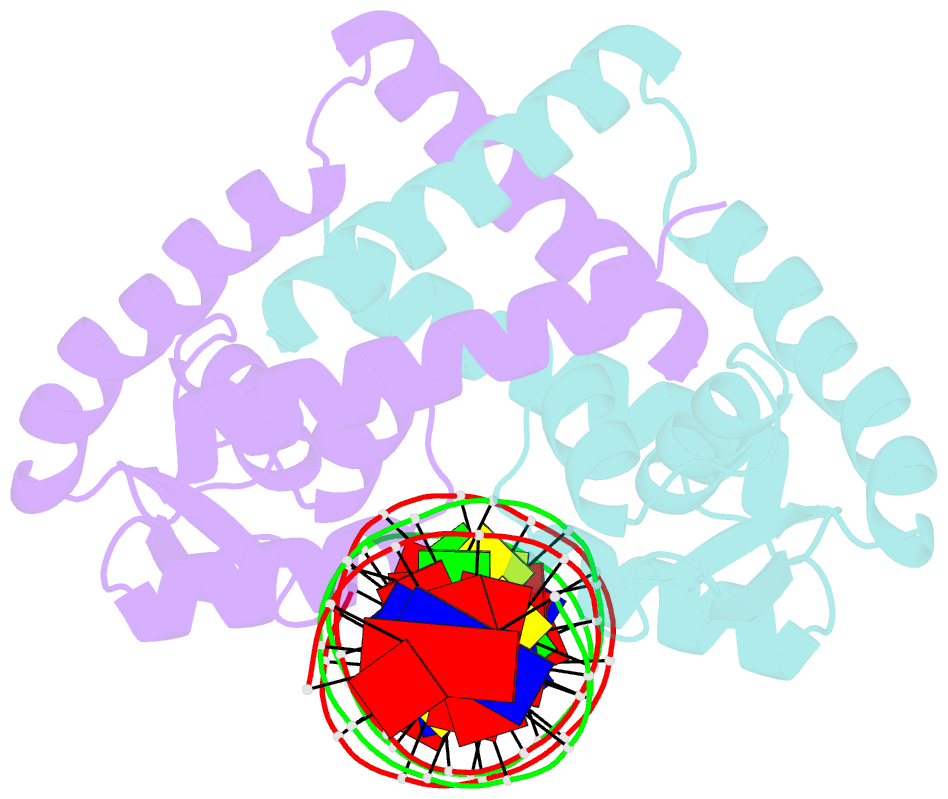
- Crystal structure of the salmonella transcriptional regulator slya in complex with DNA
- Reference
- Dolan KT, Duguid EM, He C (2011): "Crystal Structures of SlyA Protein, a Master Virulence Regulator of Salmonella, in Free and DNA-bound States." J.Biol.Chem., 286, 22178-22185. doi: 10.1074/jbc.M111.245258.
- Abstract
- SlyA is a master virulence regulator that controls the transcription of numerous genes in Salmonella enterica. We present here crystal structures of SlyA by itself and bound to a high-affinity DNA operator sequence in the slyA gene. SlyA interacts with DNA through direct recognition of a guanine base by Arg-65, as well as interactions between conserved Arg-86 and the minor groove and a large network of non-base-specific contacts with the sugar phosphate backbone. Our structures, together with an unpublished structure of SlyA bound to the small molecule effector salicylate (Protein Data Bank code 3DEU), reveal that, unlike many other MarR family proteins, SlyA dissociates from DNA without large conformational changes when bound to this effector. We propose that SlyA and other MarR global regulators rely more on indirect readout of DNA sequence to exert control over many genes, in contrast to proteins (such as OhrR) that recognize a single operator.