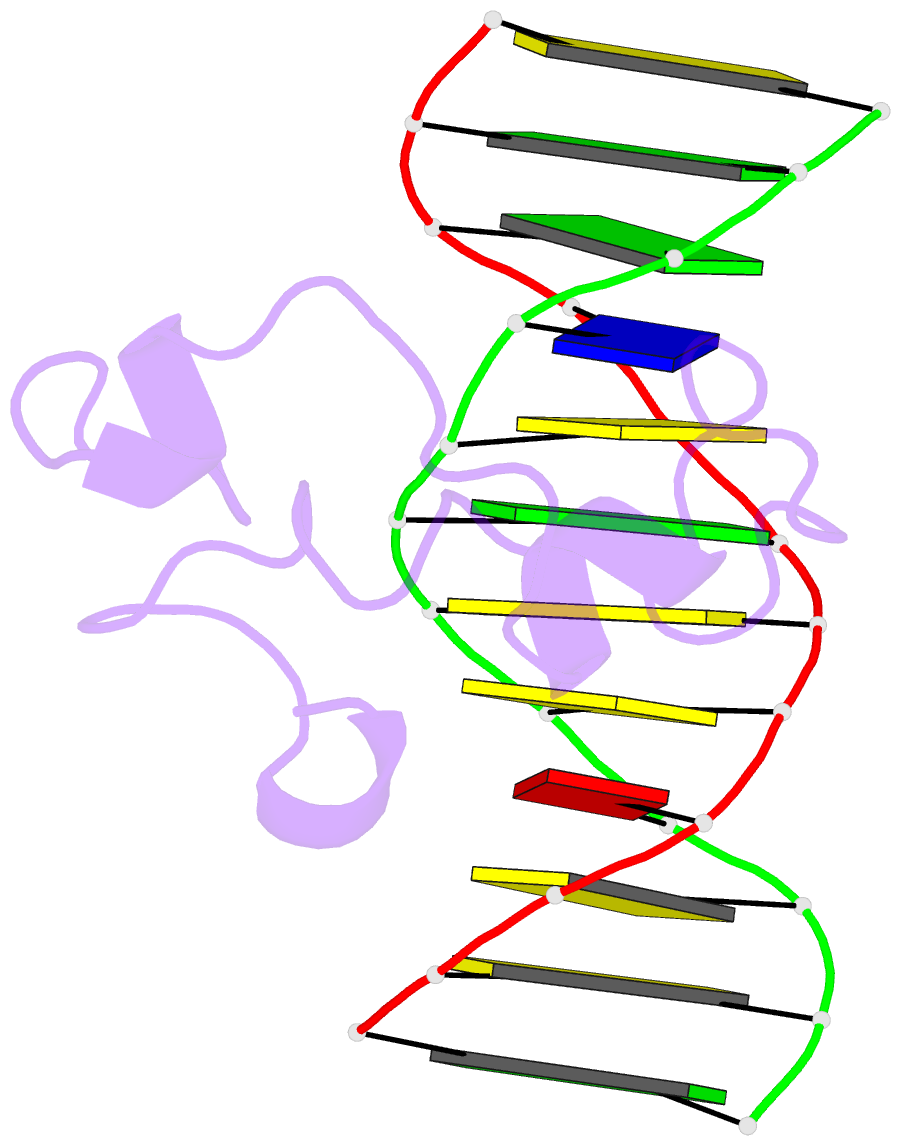
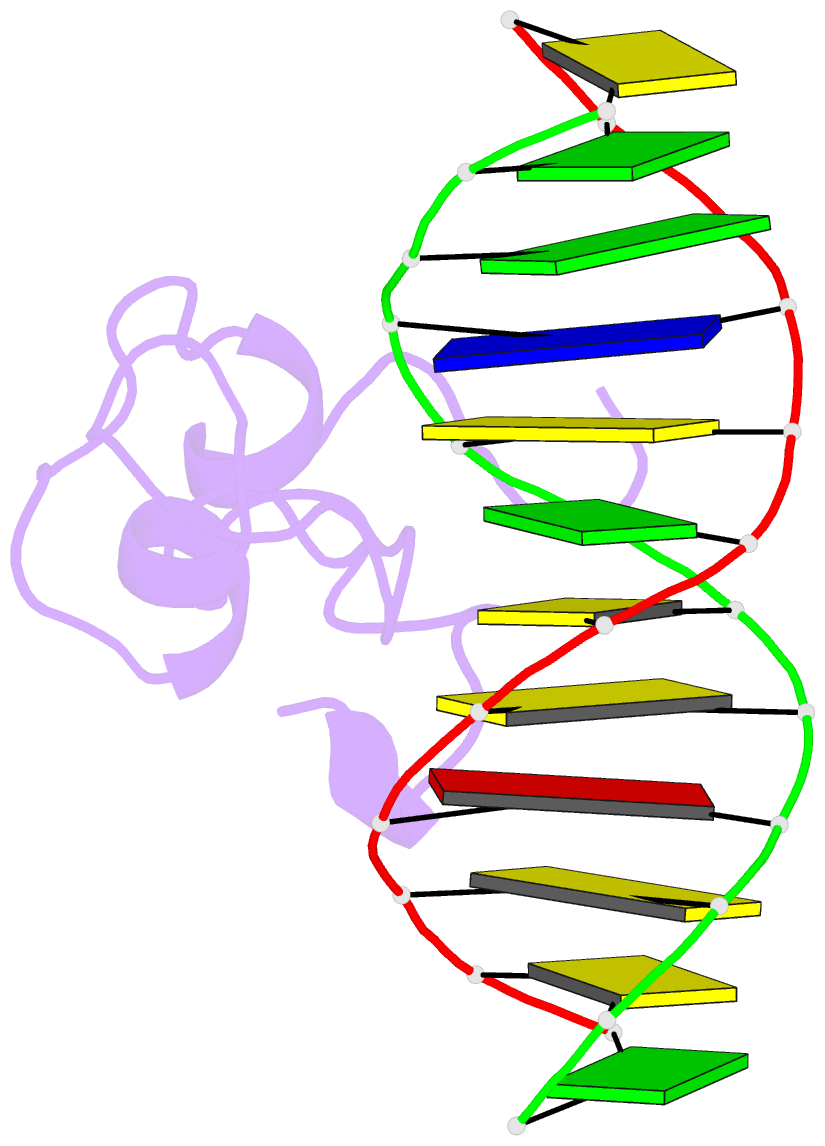
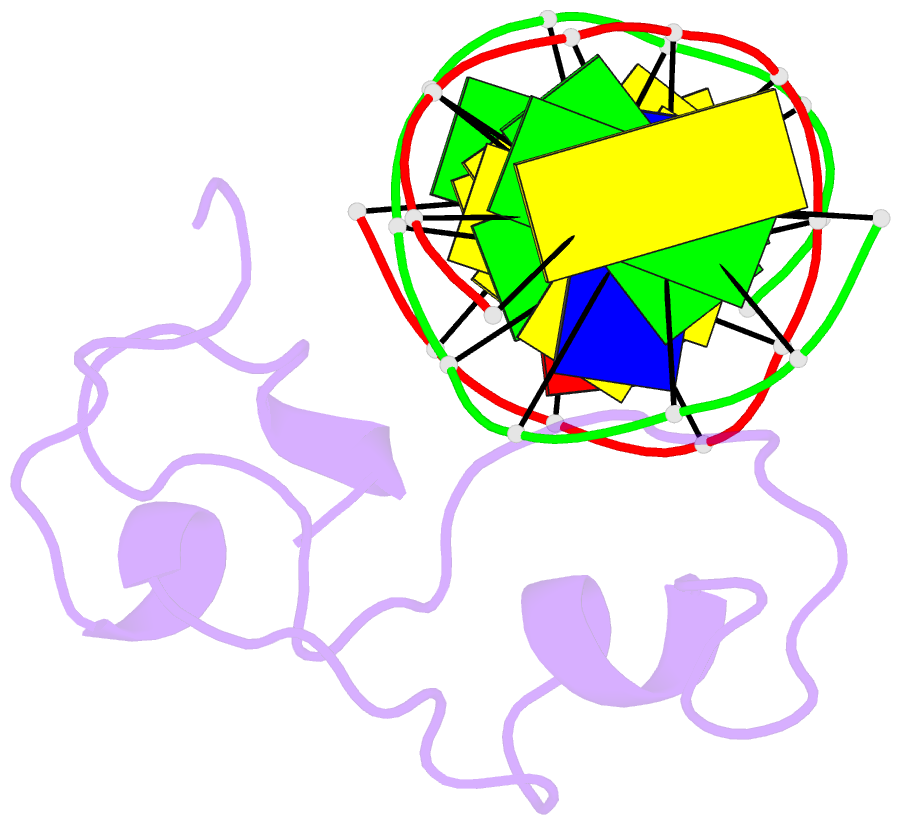
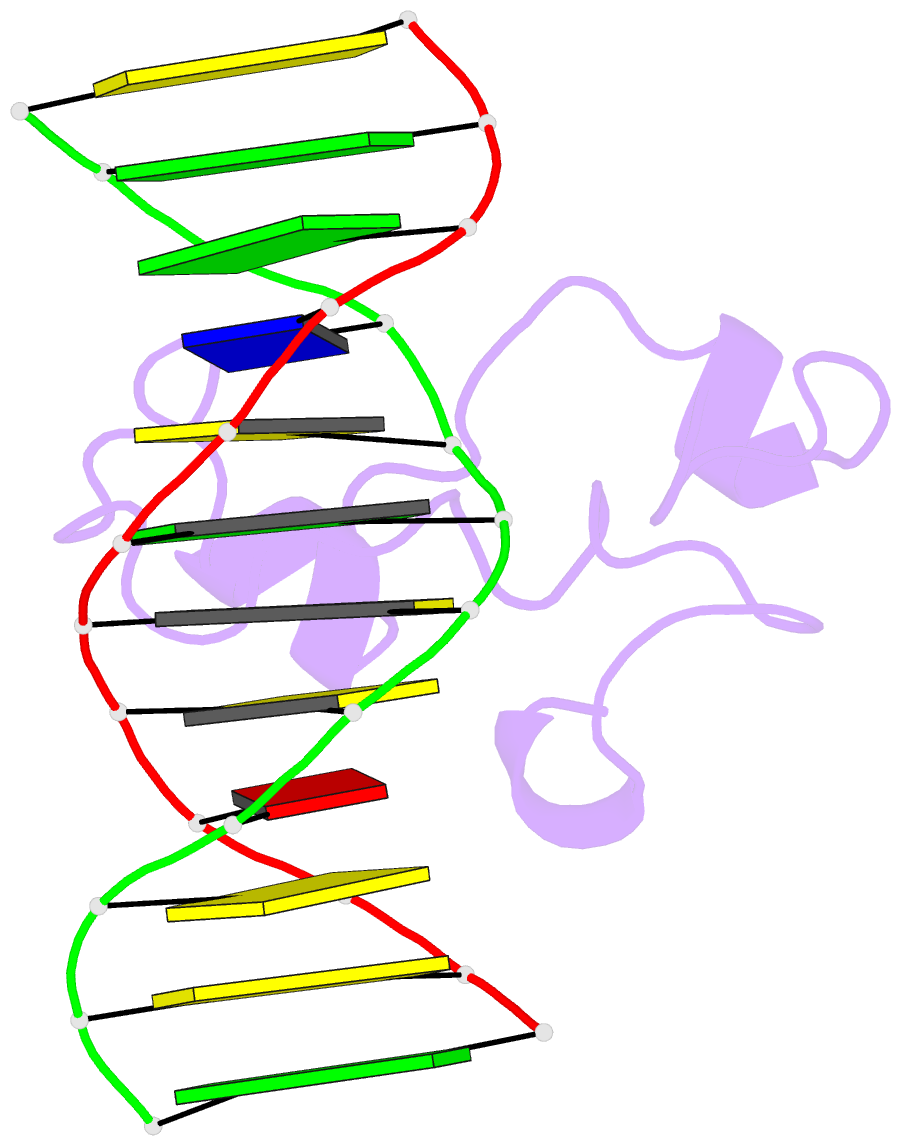
Summary information and primary citation
- PDB-id
- 3qmc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.1 Å)
- Summary
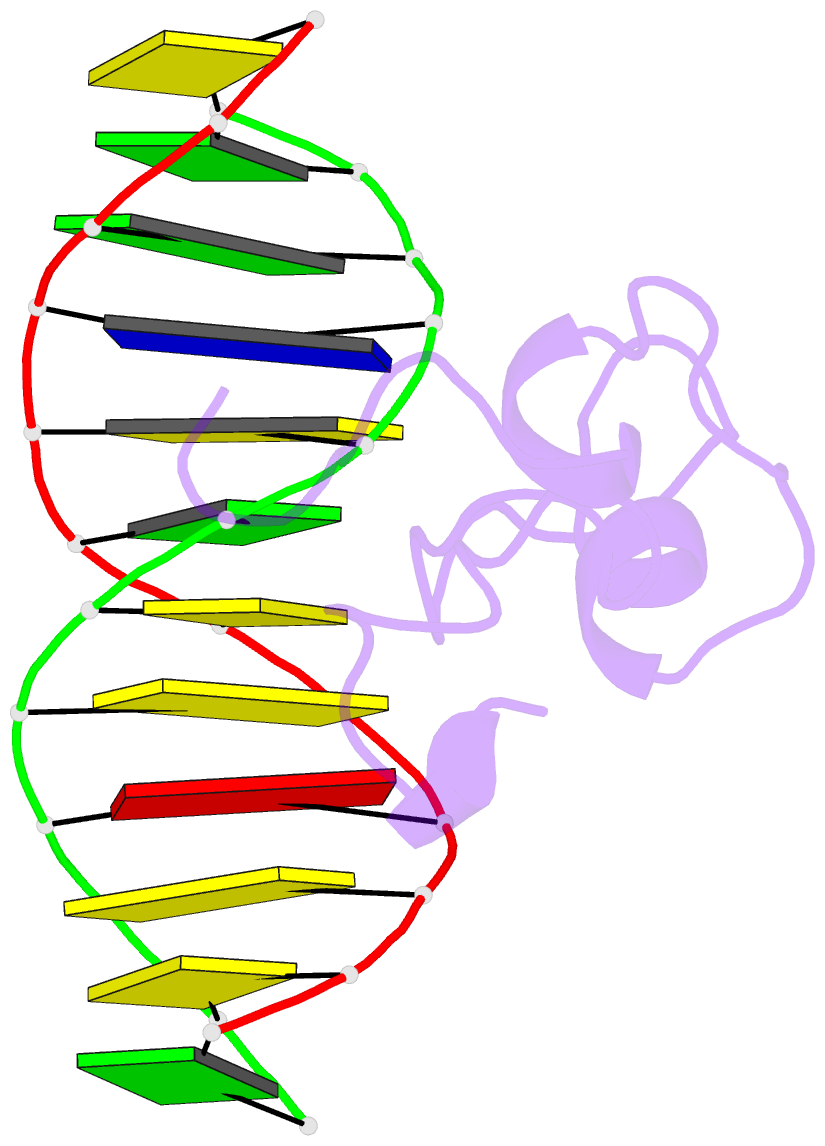
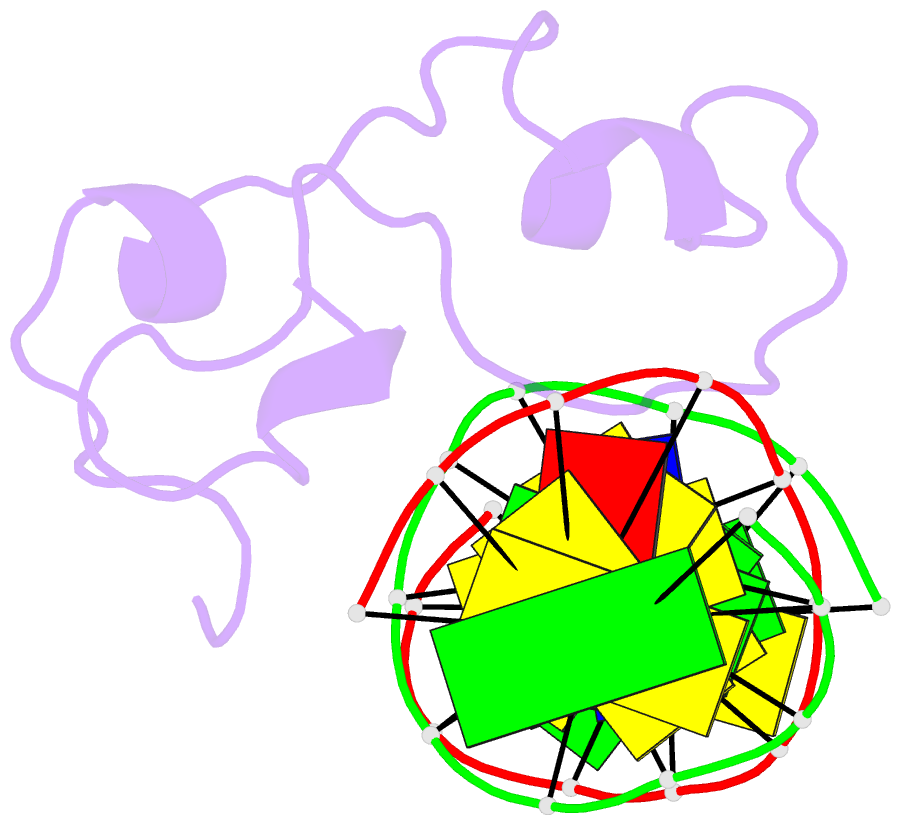
- Structural basis of selective binding of nonmethylated cpg islands by the cxxc domain of cfp1
- Reference
- Xu C, Bian C, Lam R, Dong A, Min J (2011): "The structural basis for selective binding of non-methylated CpG islands by the CFP1 CXXC domain." Nat Commun, 2, 227. doi: 10.1038/ncomms1237.
- Abstract
- CFP1 is a CXXC domain-containing protein and an essential component of the SETD1 histone H3K4 methyltransferase complex. CXXC domain proteins direct different chromatin-modifying activities to various chromatin regions. Here, we report crystal structures of the CFP1 CXXC domain in complex with six different CpG DNA sequences. The crescent-shaped CFP1 CXXC domain is wedged into the major groove of the CpG DNA, distorting the B-form DNA, and interacts extensively with the major groove of the DNA. The structures elucidate the molecular mechanism of the non-methylated CpG-binding specificity of the CFP1 CXXC domain. The CpG motif is confined by a tripeptide located in a rigid loop, which only allows the accommodation of the non-methylated CpG dinucleotide. Furthermore, we demonstrate that CFP1 has a preference for a guanosine nucleotide following the CpG motif.