Summary information and primary citation
- PDB-id
- 3qrf; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.8 Å)
- Summary
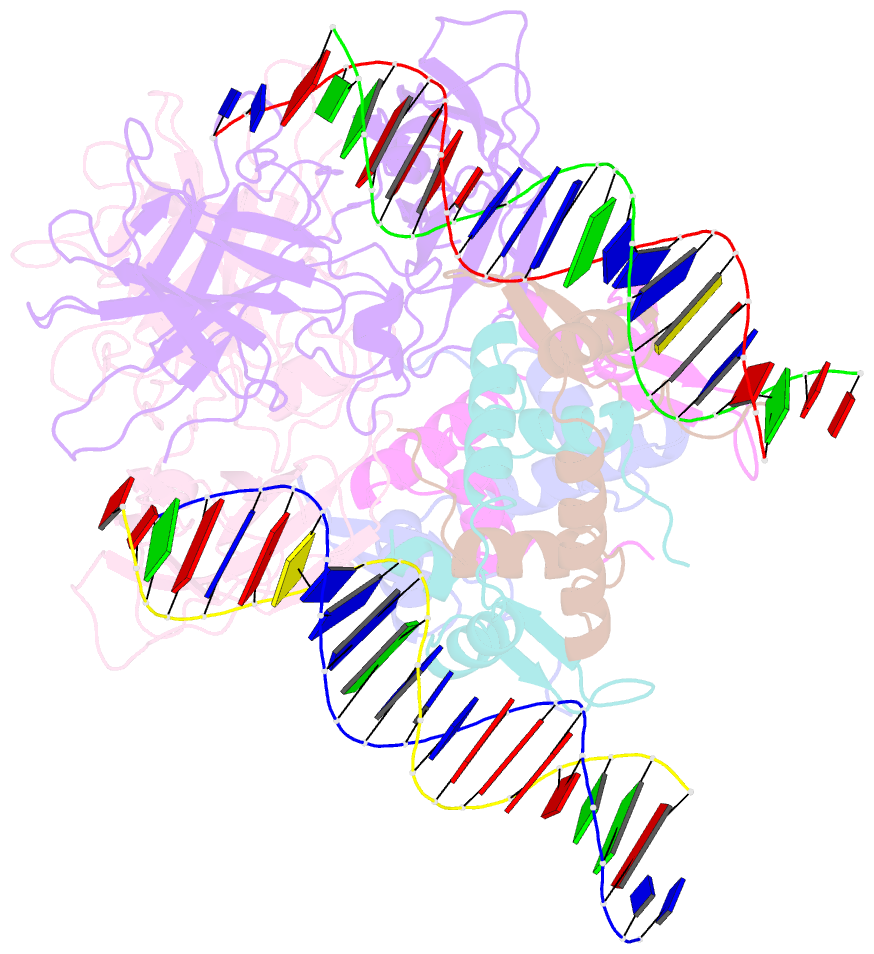
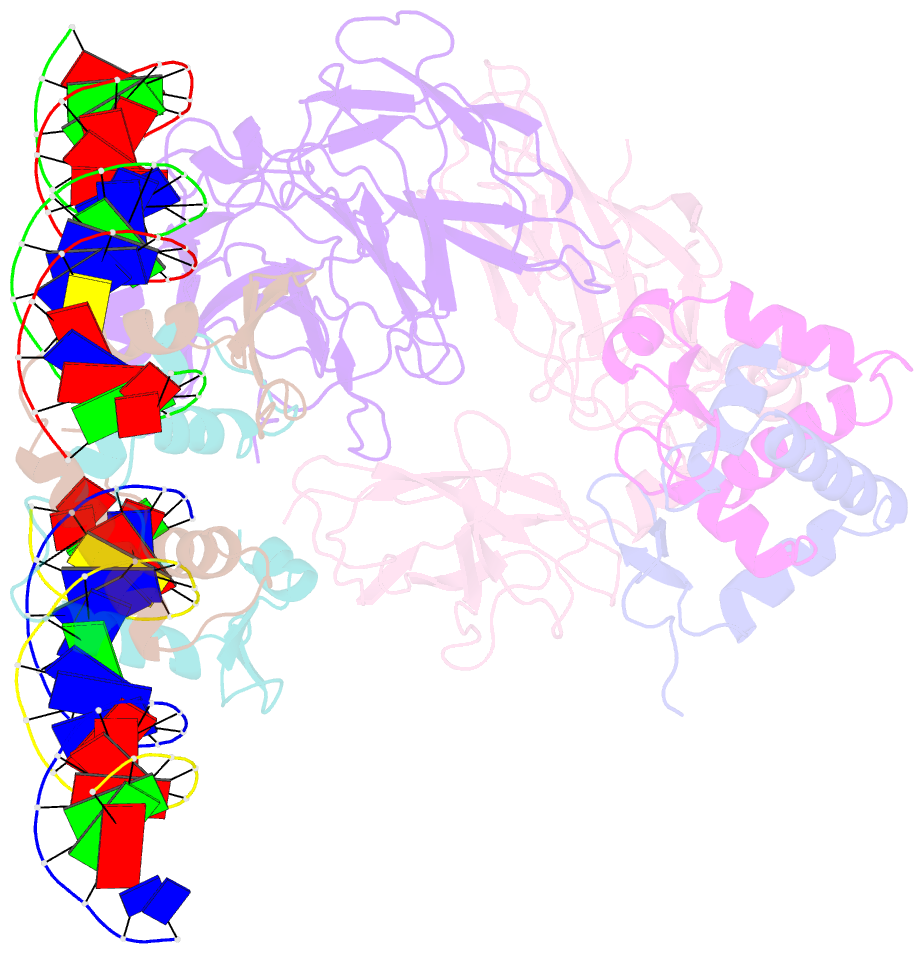
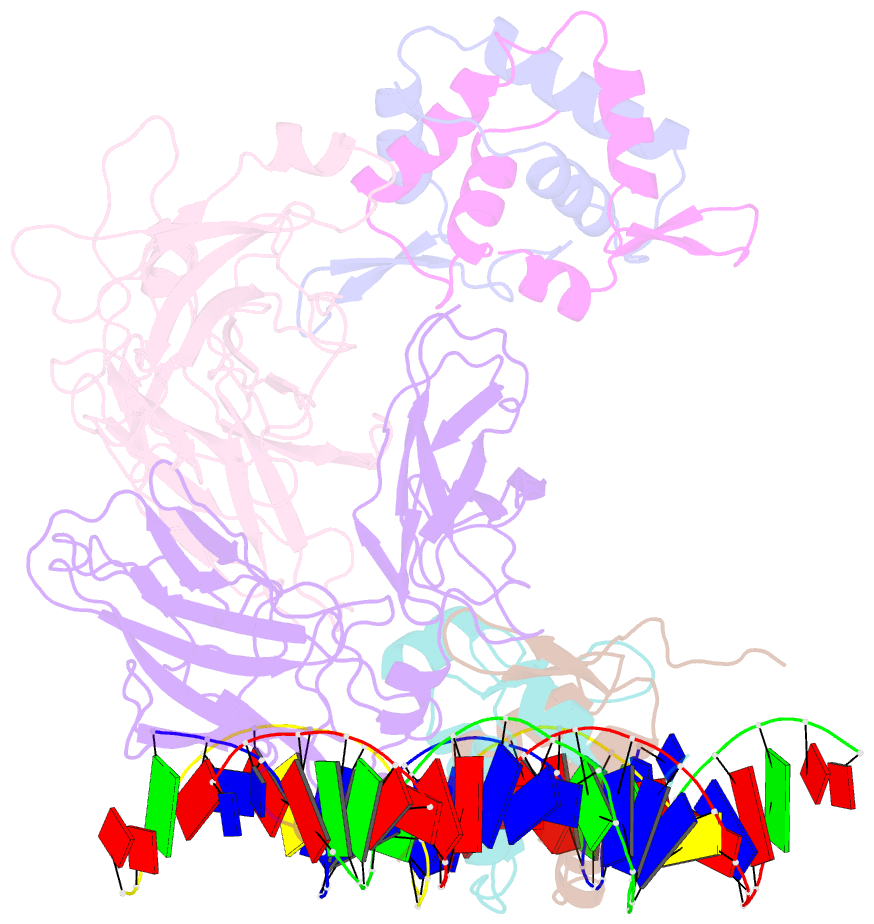
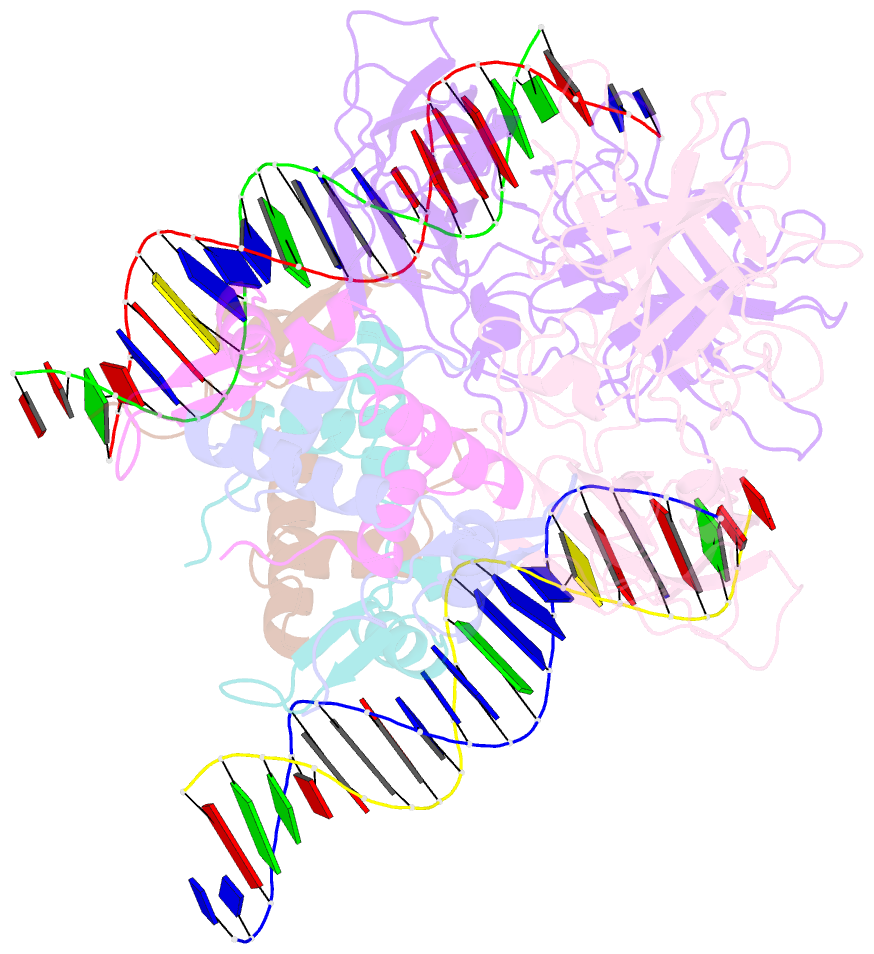
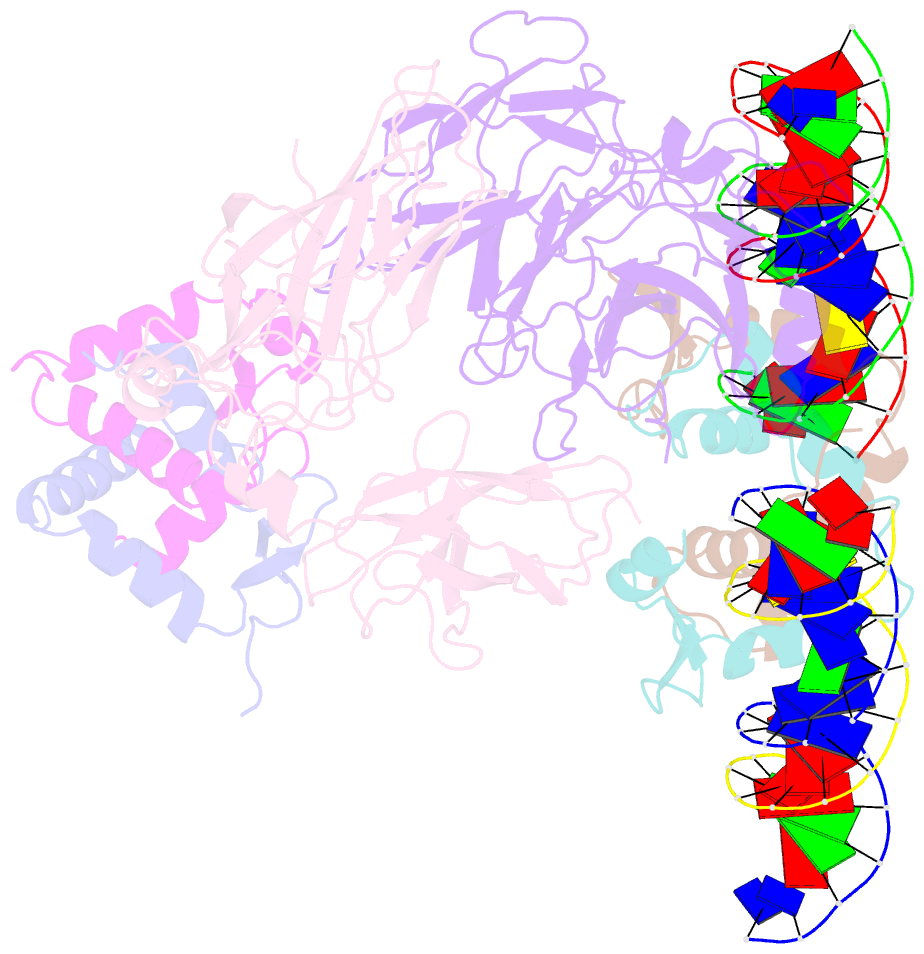
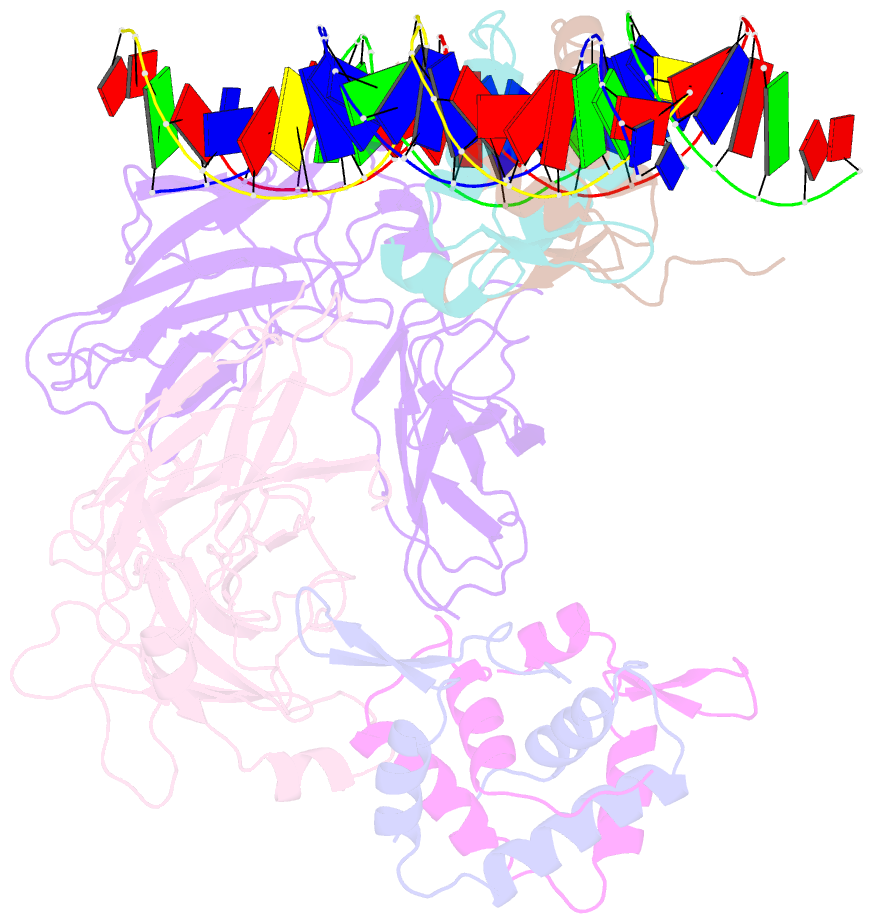
- Structure of a domain-swapped foxp3 dimer
- Reference
- Bandukwala HS, Wu Y, Feuerer M, Chen Y, Barboza B, Ghosh S, Stroud JC, Benoist C, Mathis D, Rao A, Chen L (2011): "Structure of a Domain-Swapped FOXP3 Dimer on DNA and Its Function in Regulatory T Cells." Immunity, 34, 479-491. doi: 10.1016/j.immuni.2011.02.017.
- Abstract
- The transcription factor FOXP3 is essential for the suppressive function of regulatory T cells that are required for maintaining self-tolerance. We have solved the crystal structure of the FOXP3 forkhead domain as a ternary complex with the DNA-binding domain of the transcription factor NFAT1 and a DNA oligonucleotide from the interleukin-2 promoter. A striking feature of this structure is that FOXP3 forms a domain-swapped dimer that bridges two molecules of DNA. Structure-guided or autoimmune disease (IPEX)-associated mutations in the domain-swap interface diminished dimer formation by the FOXP3 forkhead domain without compromising FOXP3 DNA binding. These mutations also eliminated T cell-suppressive activity conferred by FOXP3, both in vitro and in a murine model of autoimmune diabetes in vivo. We conclude that FOXP3-mediated suppressor function requires dimerization through the forkhead domain and that mutations in the dimer interface can lead to the systemic autoimmunity observed in IPEX patients.