Summary information and primary citation
- PDB-id
- 3qz7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (1.998 Å)
- Summary
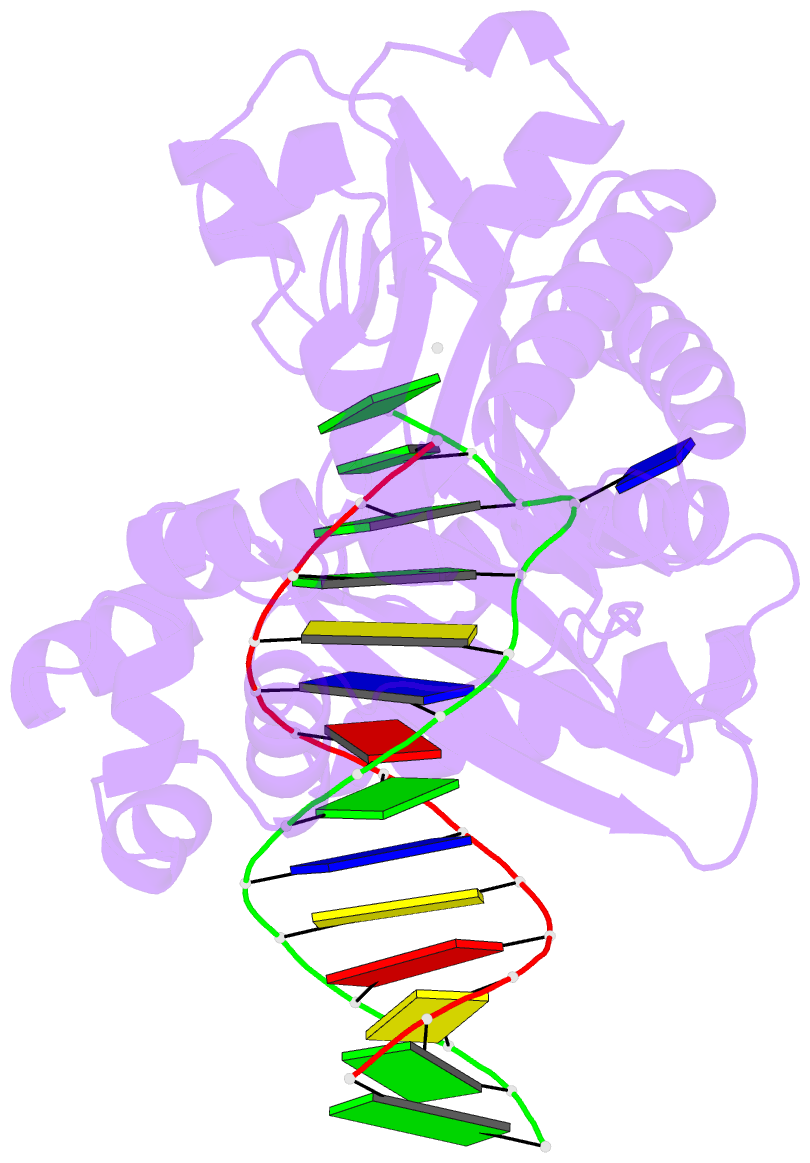
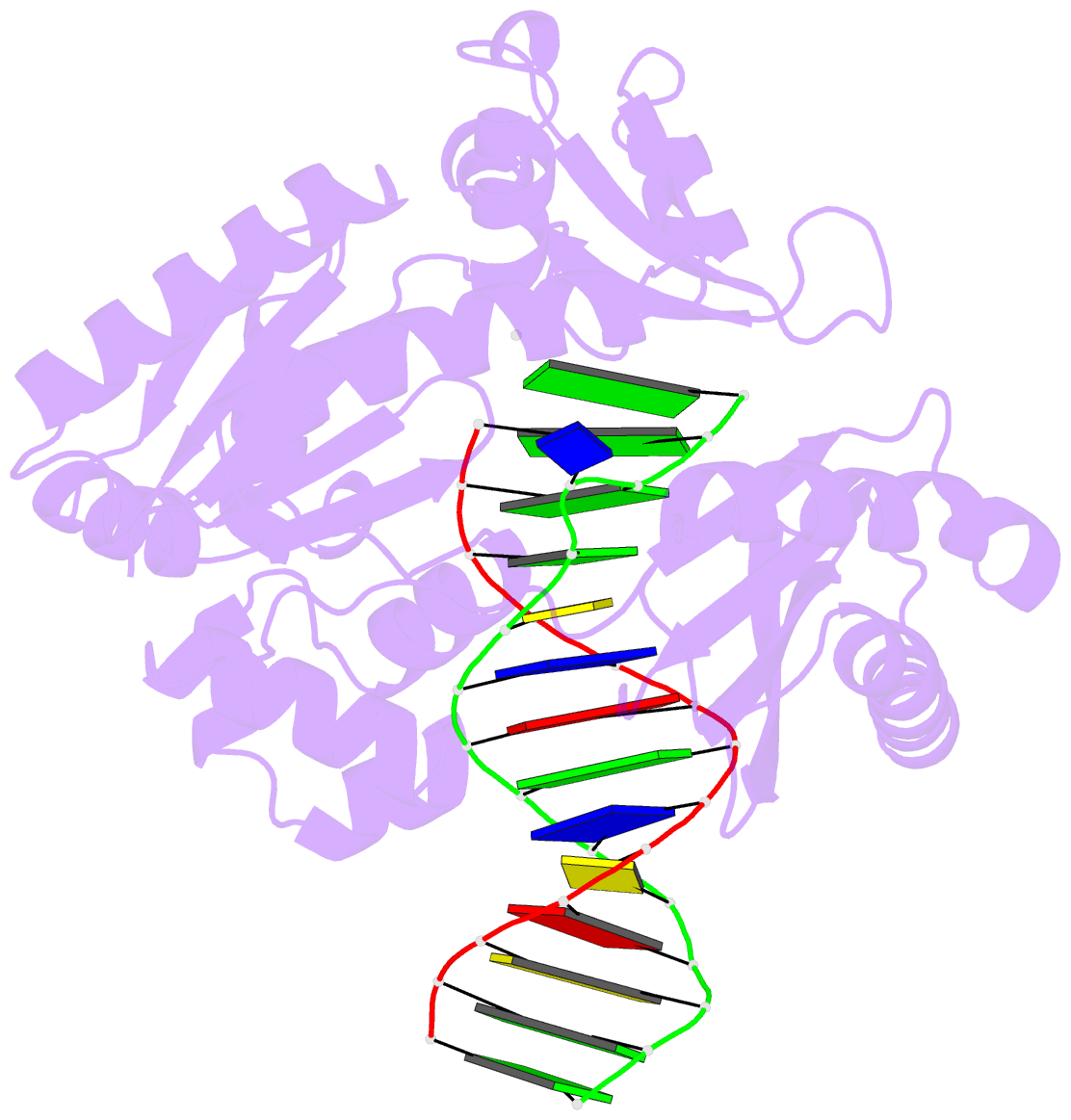
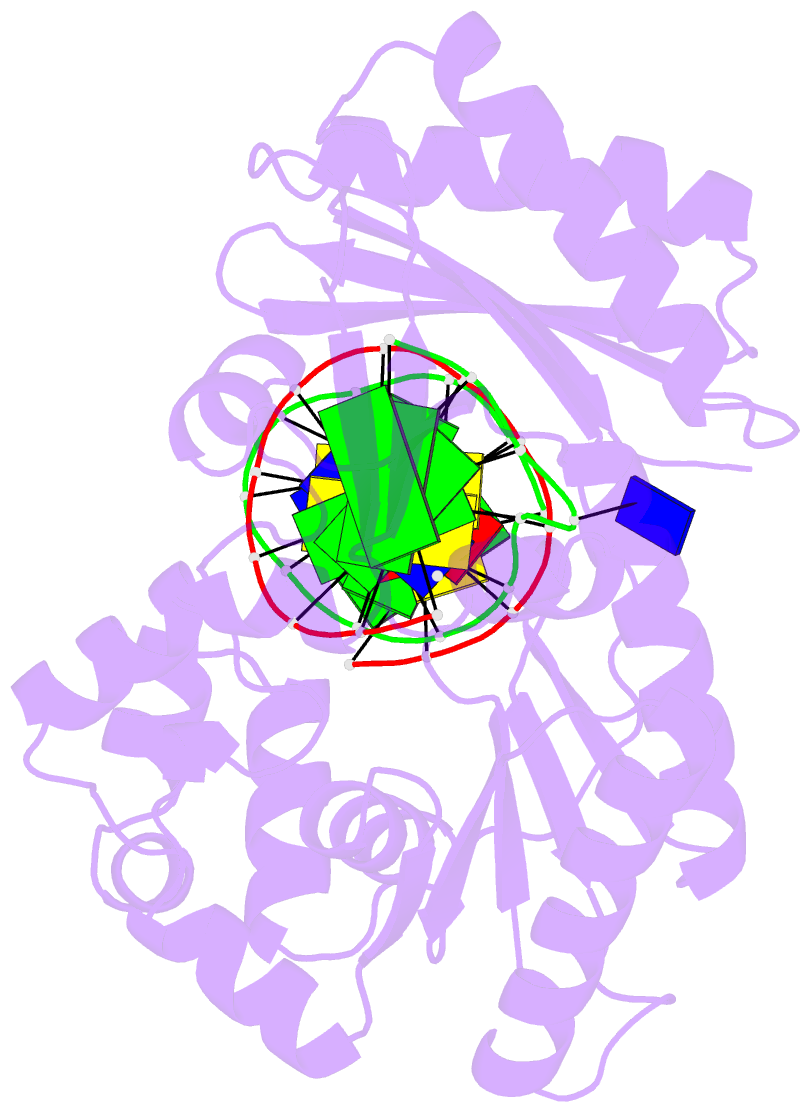
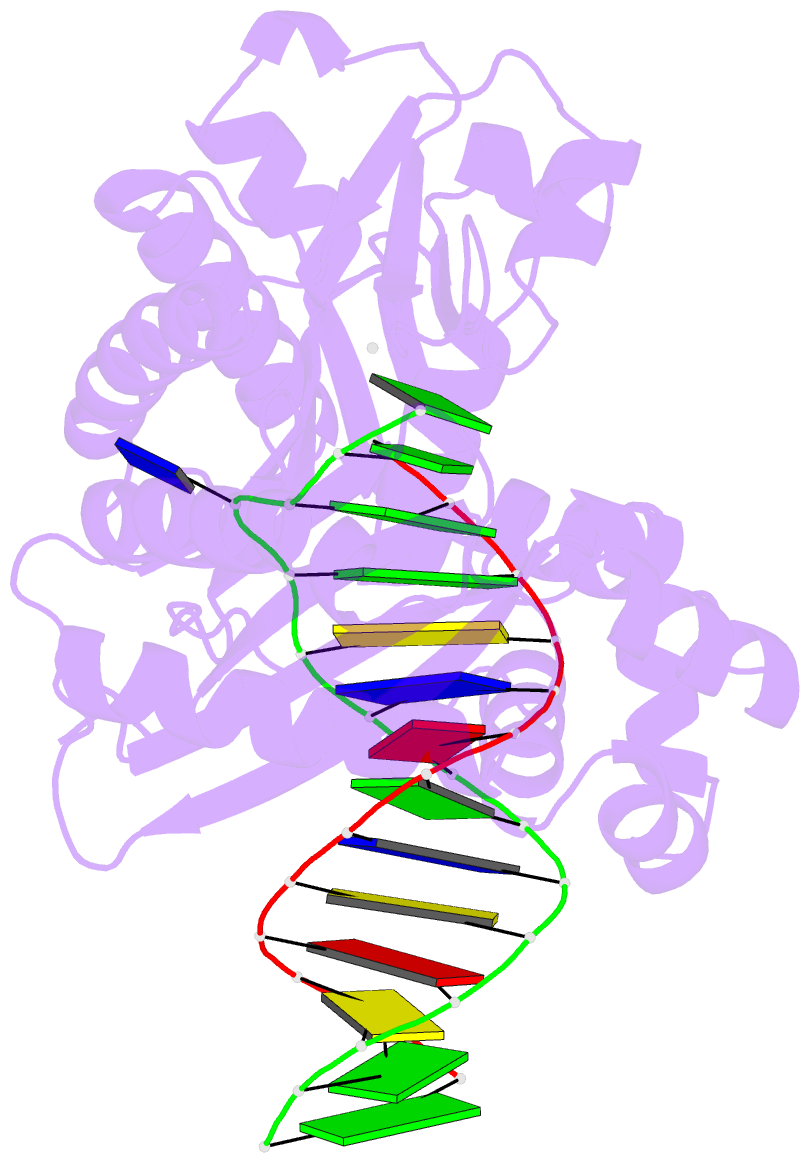
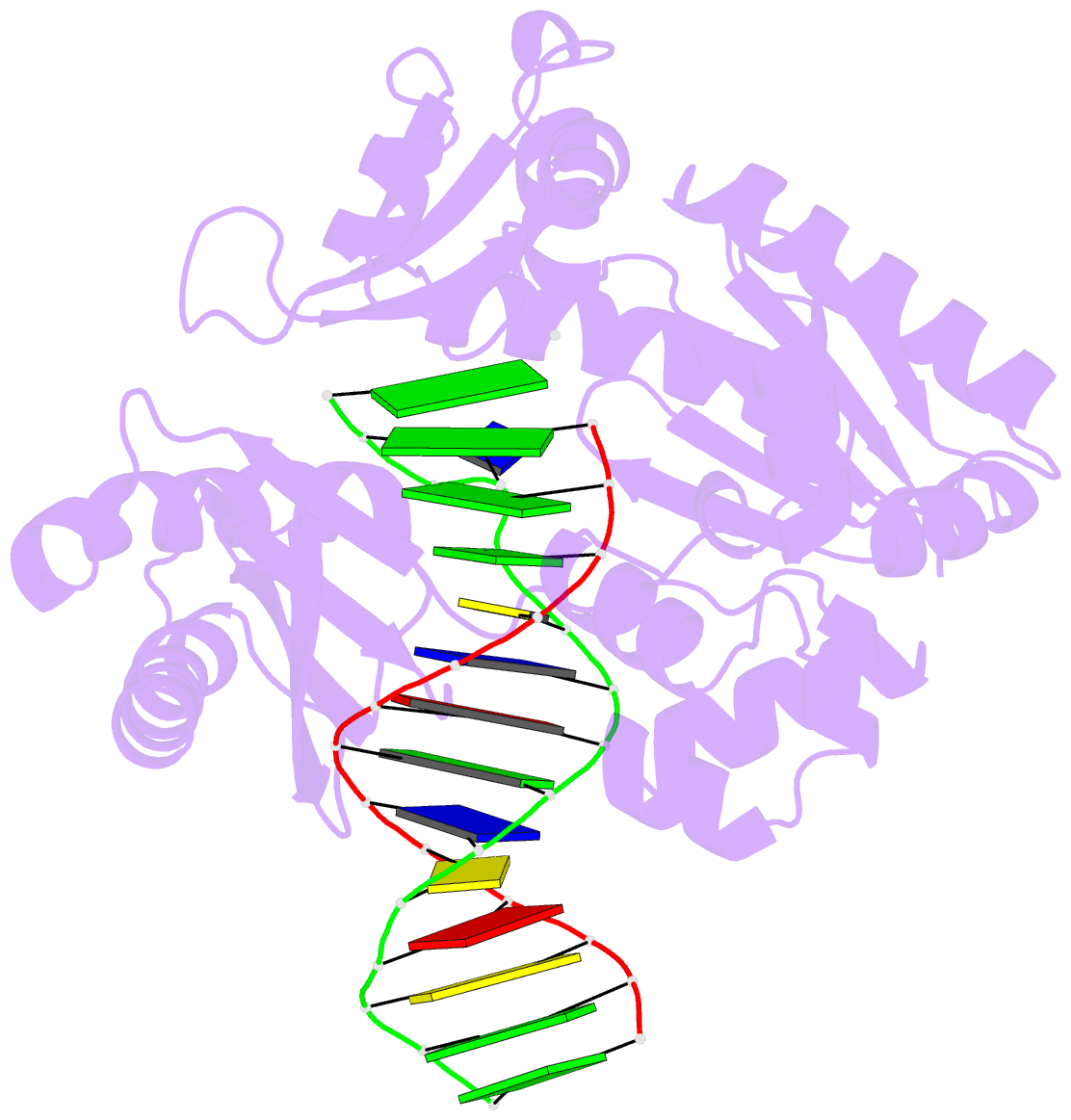
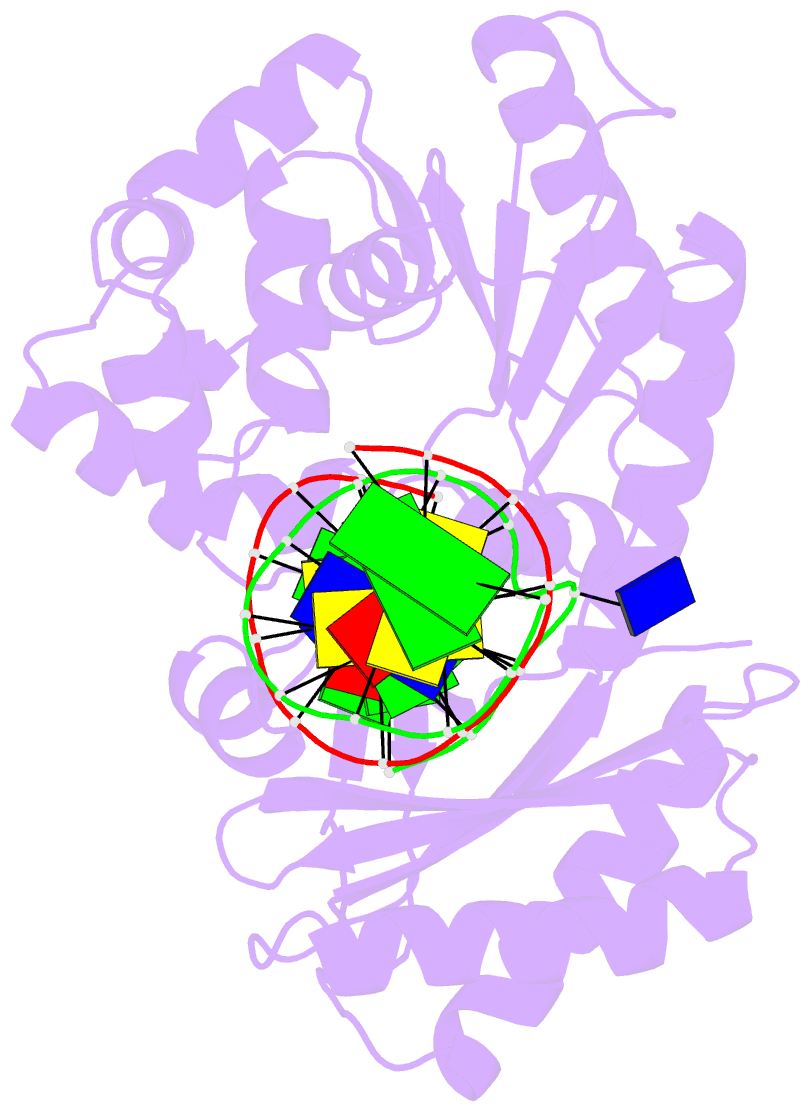
- T-3 ternary complex of dpo4
- Reference
- Wu Y, Wilson RC, Pata JD (2011): "The y-family DNA polymerase dpo4 uses a template slippage mechanism to create single-base deletions." J.Bacteriol., 193, 2630-2636. doi: 10.1128/JB.00012-11.
- Abstract
- The Y-family polymerases help cells tolerate DNA damage by performing translesion synthesis, yet they also can be highly error prone. One distinctive feature of the DinB class of Y-family polymerases is that they make single-base deletion errors at high frequencies in repetitive sequences, especially those that contain two or more identical pyrimidines with a 5' flanking guanosine. Intriguingly, different deletion mechanisms have been proposed, even for two archaeal DinB polymerases that share 54% sequence identity and originate from two strains of Sulfolobus. To reconcile these apparent differences, we have characterized Dpo4 from Sulfolobus solfataricus using the same biochemical and crystallographic approaches that we have used previously to characterize Dbh from Sulfolobus acidocaldarius. In contrast to previous suggestions that Dpo4 uses a deoxynucleoside triphosphate (dNTP)-stabilized misalignment mechanism when creating single-base deletions, we find that Dpo4 predominantly uses a template slippage deletion mechanism when replicating repetitive DNA sequences, as was previously shown for Dbh. Dpo4 stabilizes the skipped template base in an extrahelical conformation between the polymerase and the little-finger domains of the enzyme. This contrasts with Dbh, in which the extrahelical base is stabilized against the surface of the little-finger domain alone. Thus, despite sharing a common deletion mechanism, these closely related polymerases use different contacts with the substrate to accomplish the same result.