Summary information and primary citation
- PDB-id
- 3r1h; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (3.15 Å)
- Summary
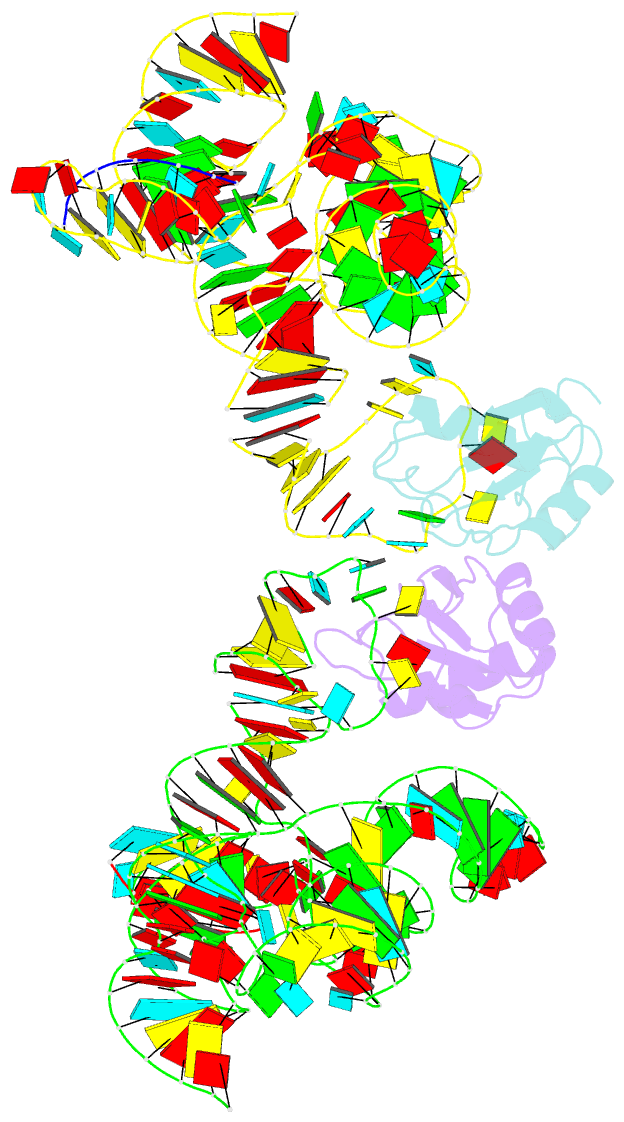
- Crystal structure of the class i ligase ribozyme-substrate preligation complex, c47u mutant, ca2+ bound
- Reference
- Shechner DM, Bartel DP (2011): "The structural basis of RNA-catalyzed RNA polymerization." Nat.Struct.Mol.Biol., 18, 1036-1042. doi: 10.1038/nsmb.2107.
- Abstract
- Early life presumably required polymerase ribozymes capable of replicating RNA. Known polymerase ribozymes best approximating such replicases use as their catalytic engine an RNA-ligase ribozyme originally selected from random RNA sequences. Here we report 3.15-Å crystal structures of this ligase trapped in catalytically viable preligation states, with the 3'-hydroxyl nucleophile positioned for in-line attack on the 5'-triphosphate. Guided by metal- and solvent-mediated interactions, the 5'-triphosphate hooks into the major groove of the adjoining RNA duplex in an unanticipated conformation. Two phosphates and the nucleophile jointly coordinate an active-site metal ion. Atomic mutagenesis experiments demonstrate that active-site nucleobase and hydroxyl groups also participate directly in catalysis, collectively playing a role that in proteinaceous polymerases is performed by a second metal ion. Thus artificial ribozymes can use complex catalytic strategies that differ markedly from those of analogous biological enzymes.