Summary information and primary citation
- PDB-id
- 3r8f; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication activator-DNA
- Method
- X-ray (3.366 Å)
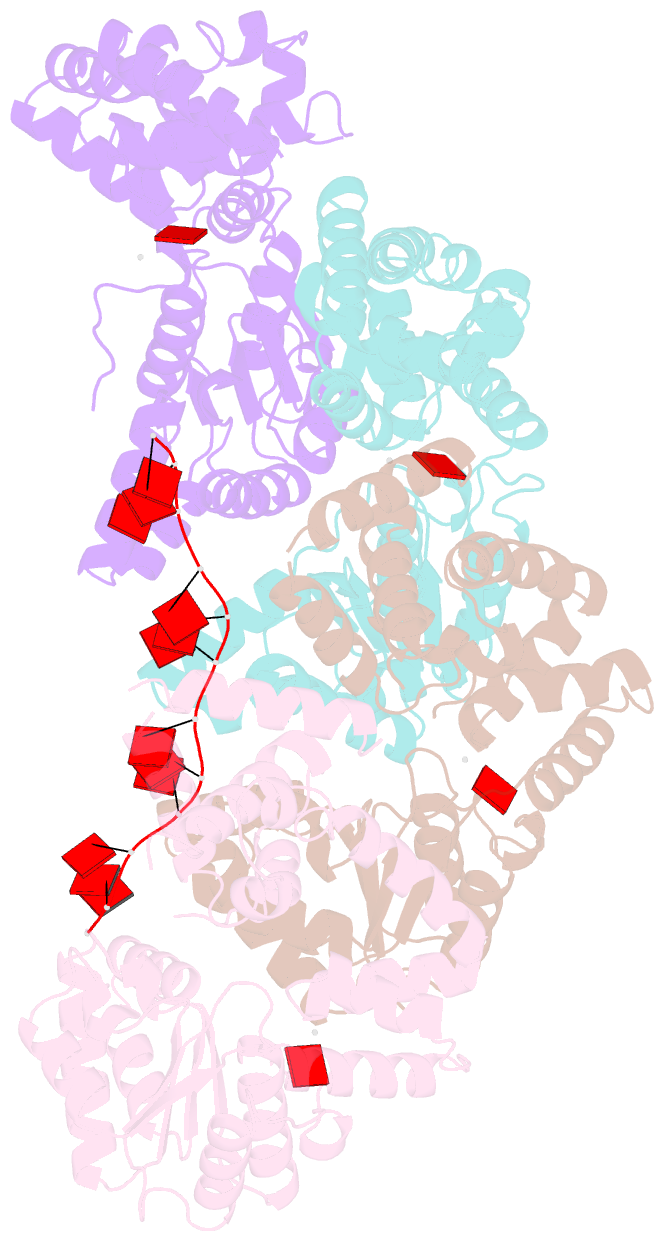
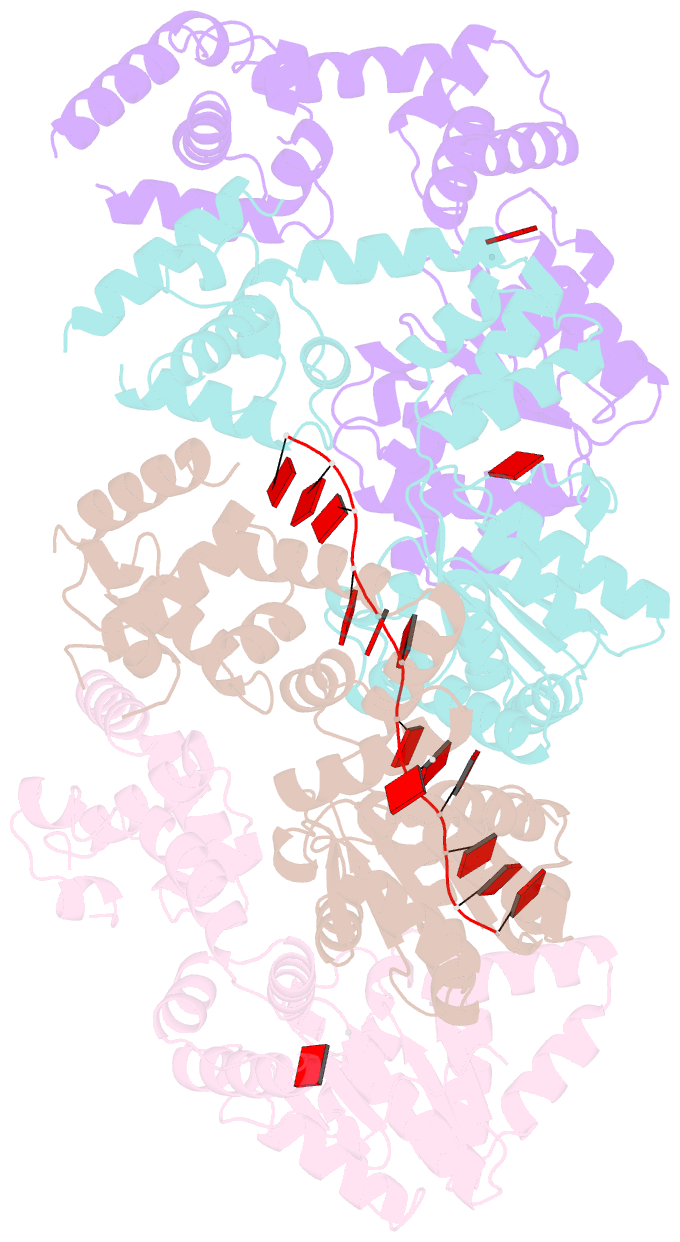
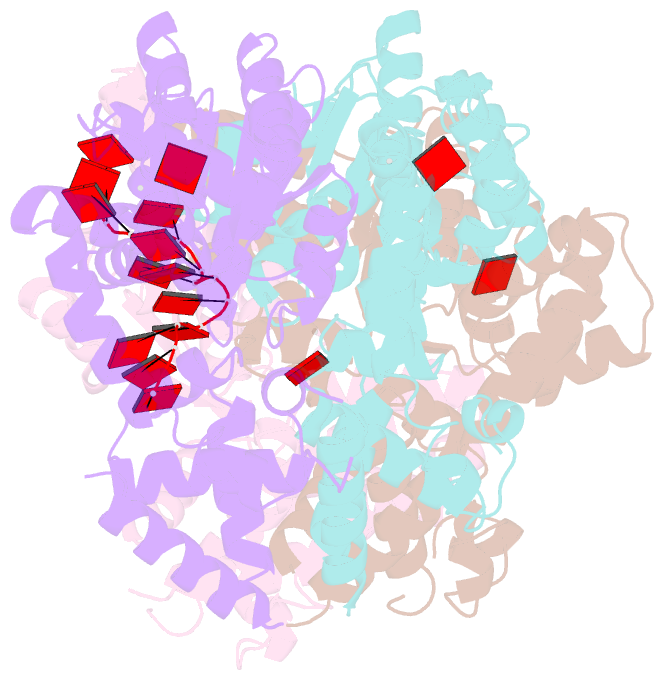
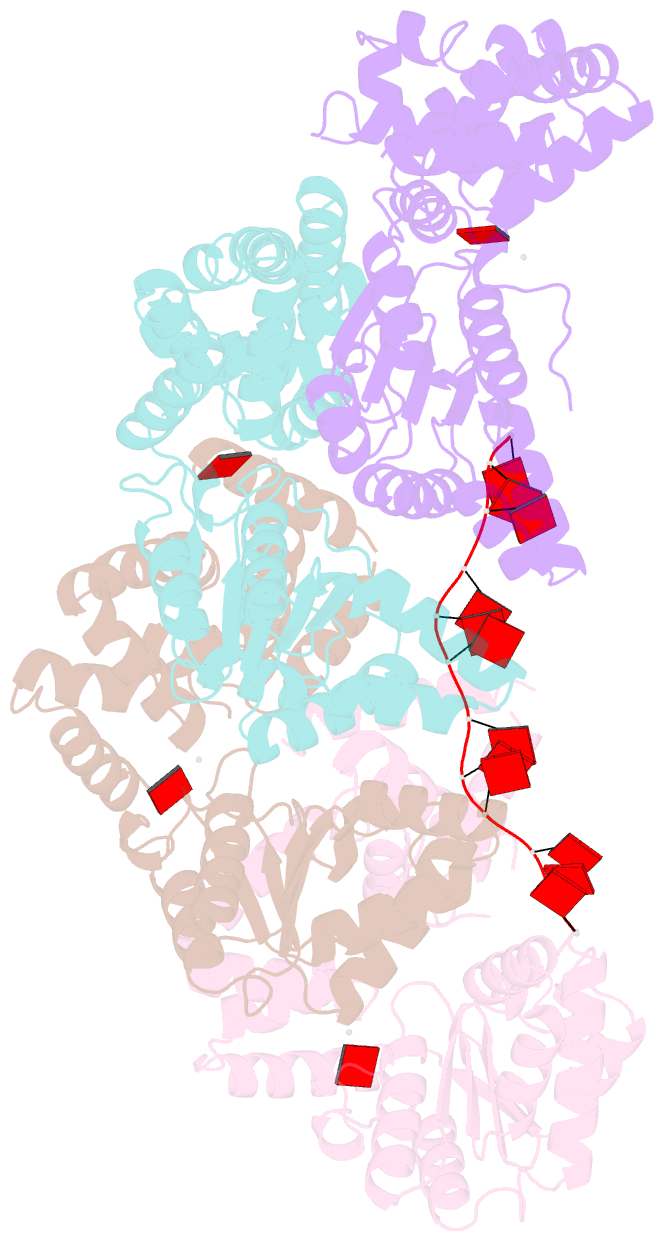
- Summary
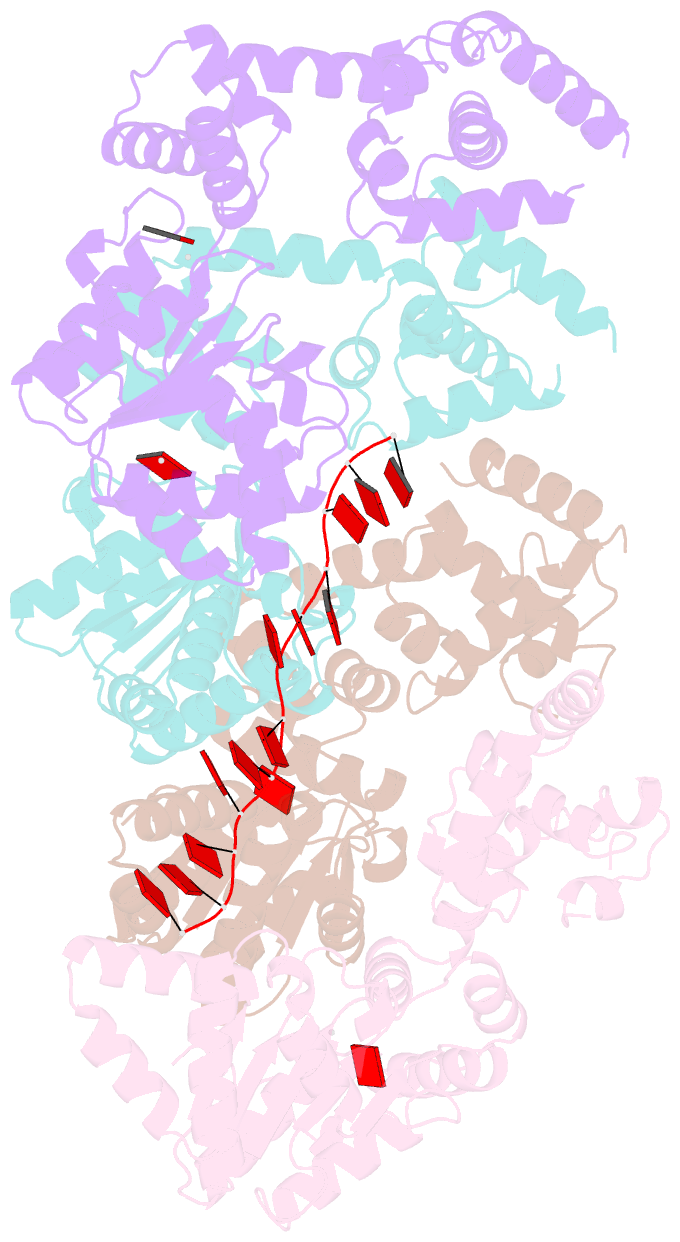
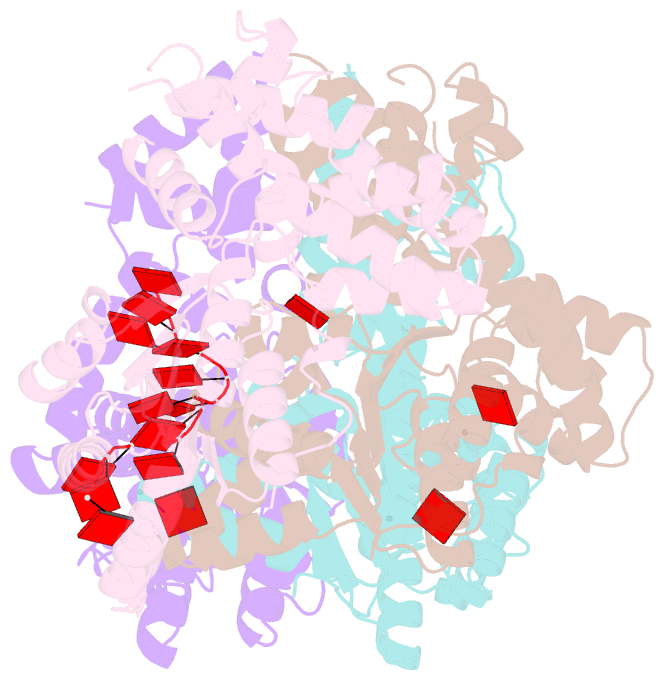
- Replication initiator dnaa bound to amppcp and single-stranded DNA
- Reference
- Duderstadt KE, Chuang K, Berger JM (2011): "DNA stretching by bacterial initiators promotes replication origin opening." Nature, 478, 209-213. doi: 10.1038/nature10455.
- Abstract
- Many replication initiators form higher-order oligomers that process host replication origins to promote replisome formation. In addition to dedicated duplex-DNA-binding domains, cellular initiators possess AAA+ (ATPases associated with various cellular activities) elements that drive functions ranging from protein assembly to origin recognition. In bacteria, the AAA+ domain of the initiator DnaA has been proposed to assist in single-stranded DNA formation during origin melting. Here we show crystallographically and in solution that the ATP-dependent assembly of Aquifex aeolicus DnaA into a spiral oligomer creates a continuous surface that allows successive AAA+ domains to bind and extend single-stranded DNA segments. The mechanism of binding is unexpectedly similar to that of RecA, a homologous recombination factor, but it differs in that DnaA promotes a nucleic acid conformation that prevents pairing of a complementary strand. These findings, combined with strand-displacement assays, indicate that DnaA opens replication origins by a direct ATP-dependent stretching mechanism. Comparative studies reveal notable commonalities between the approach used by DnaA to engage DNA substrates and other, nucleic-acid-dependent, AAA+ systems.