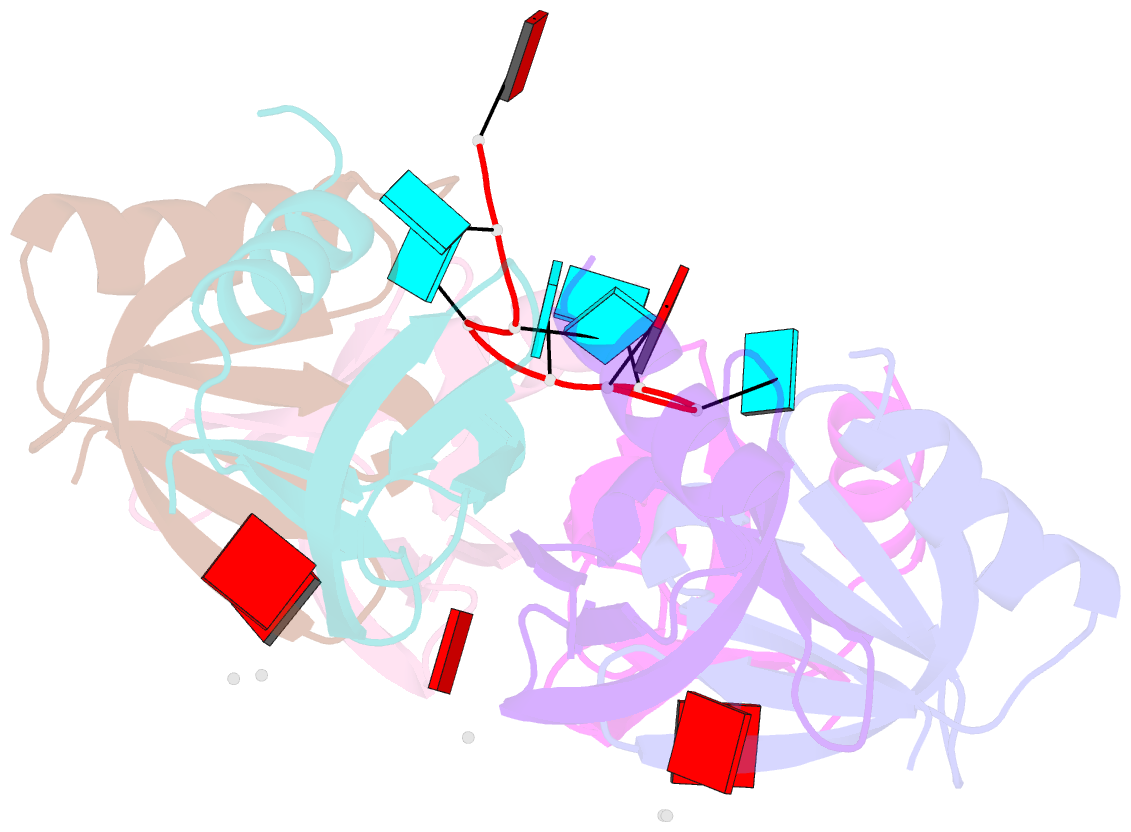
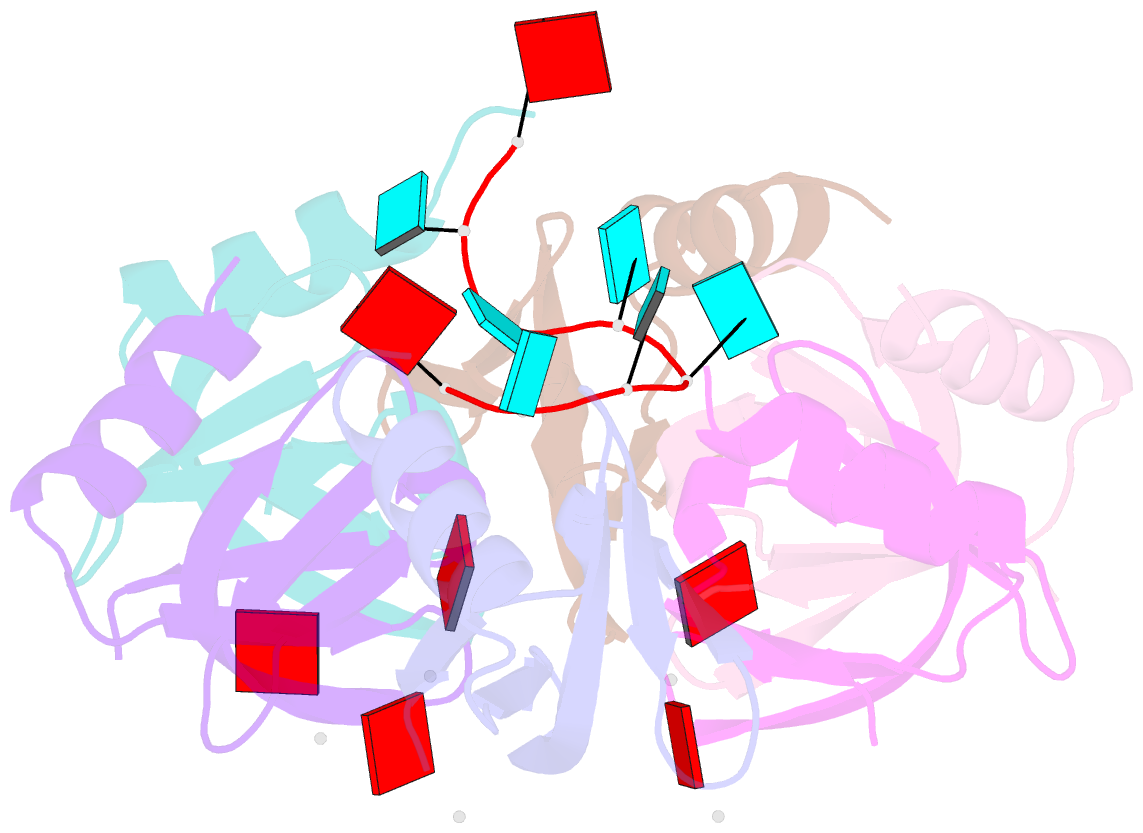
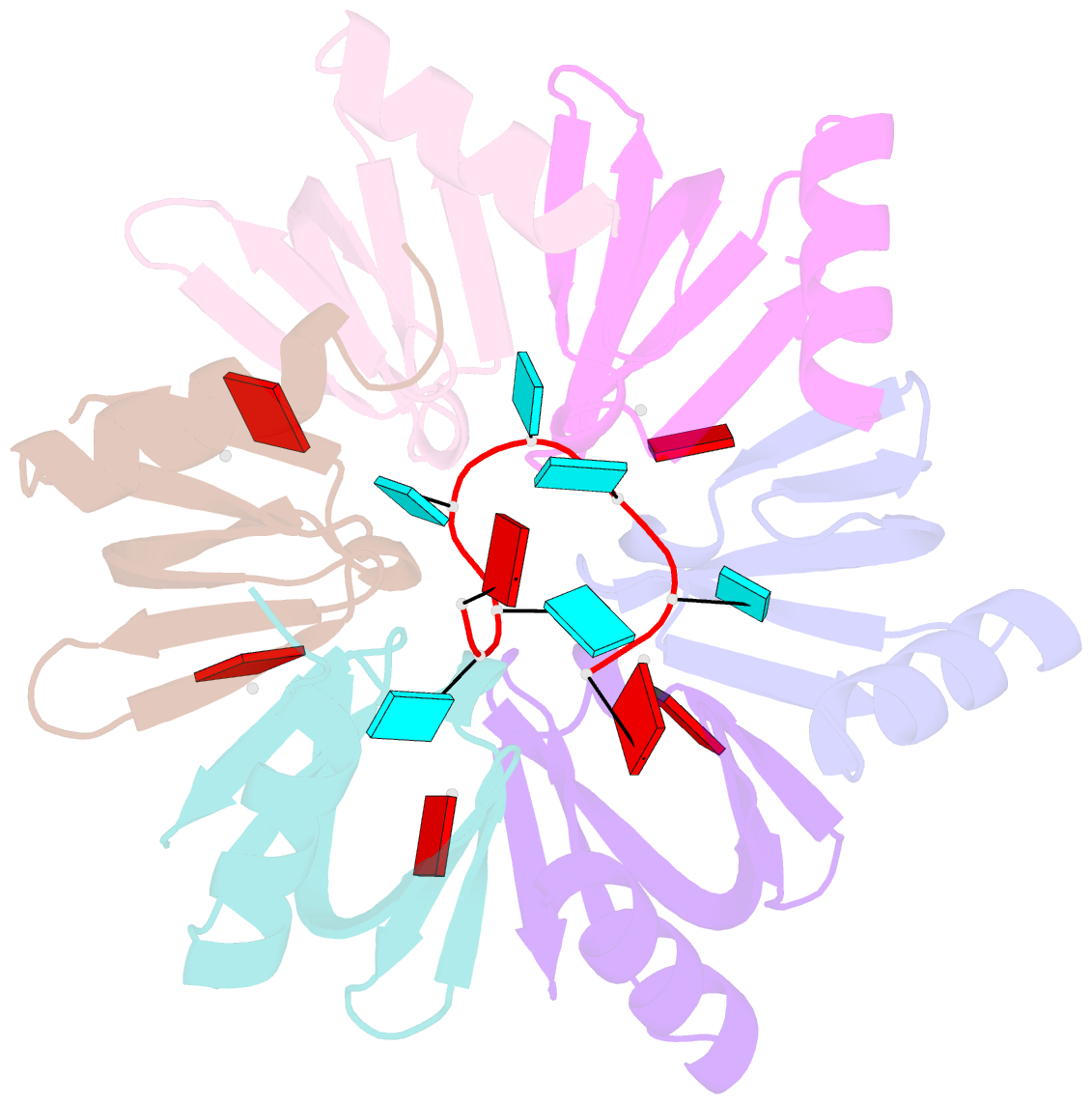
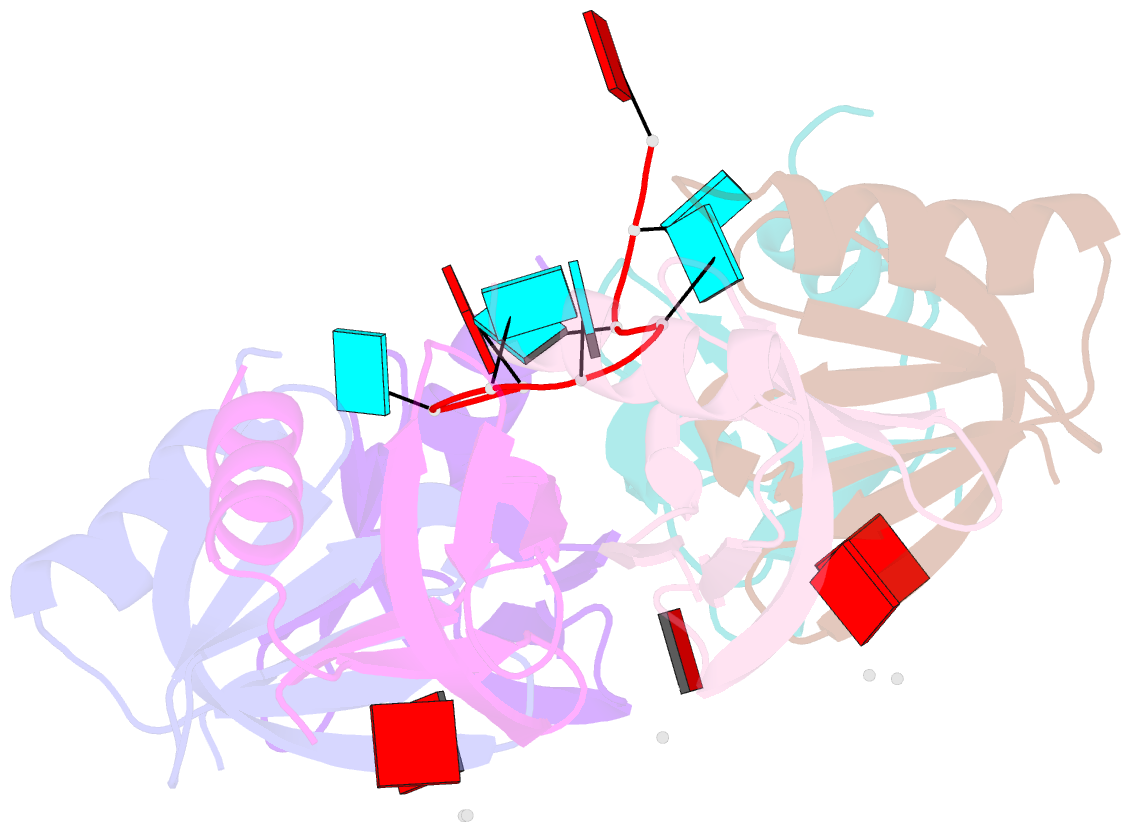
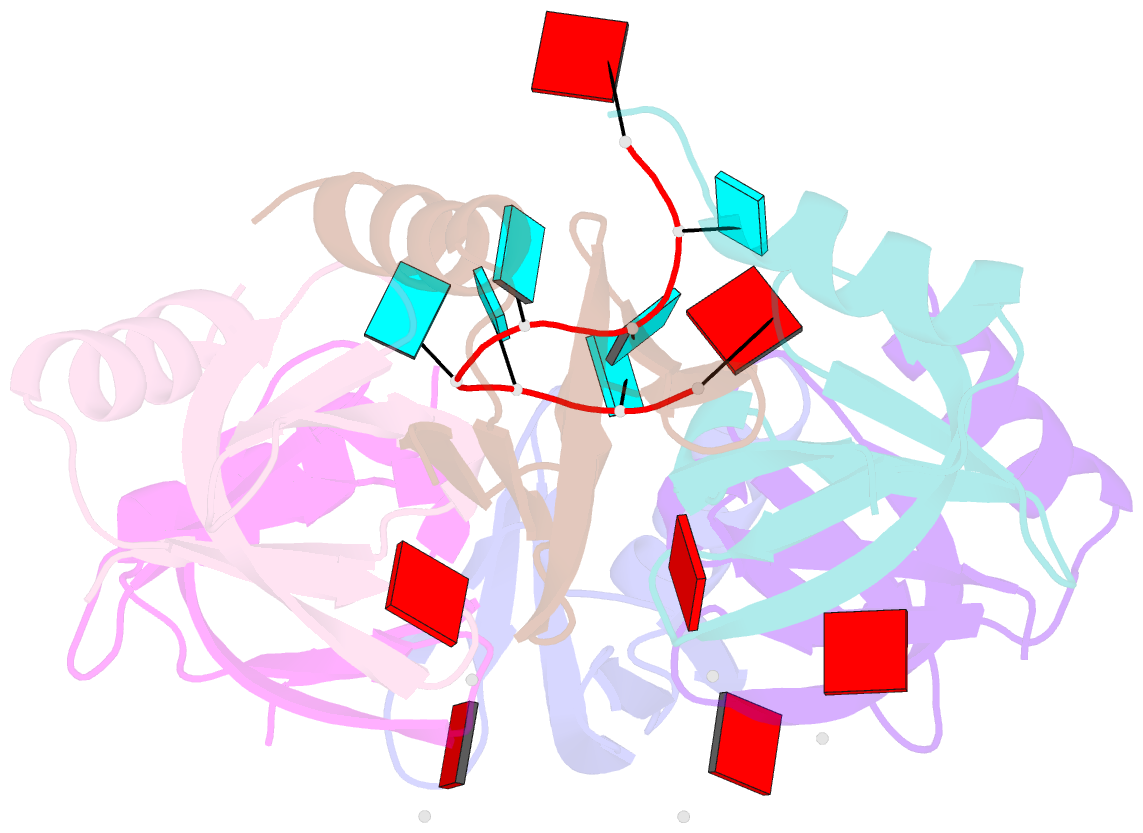
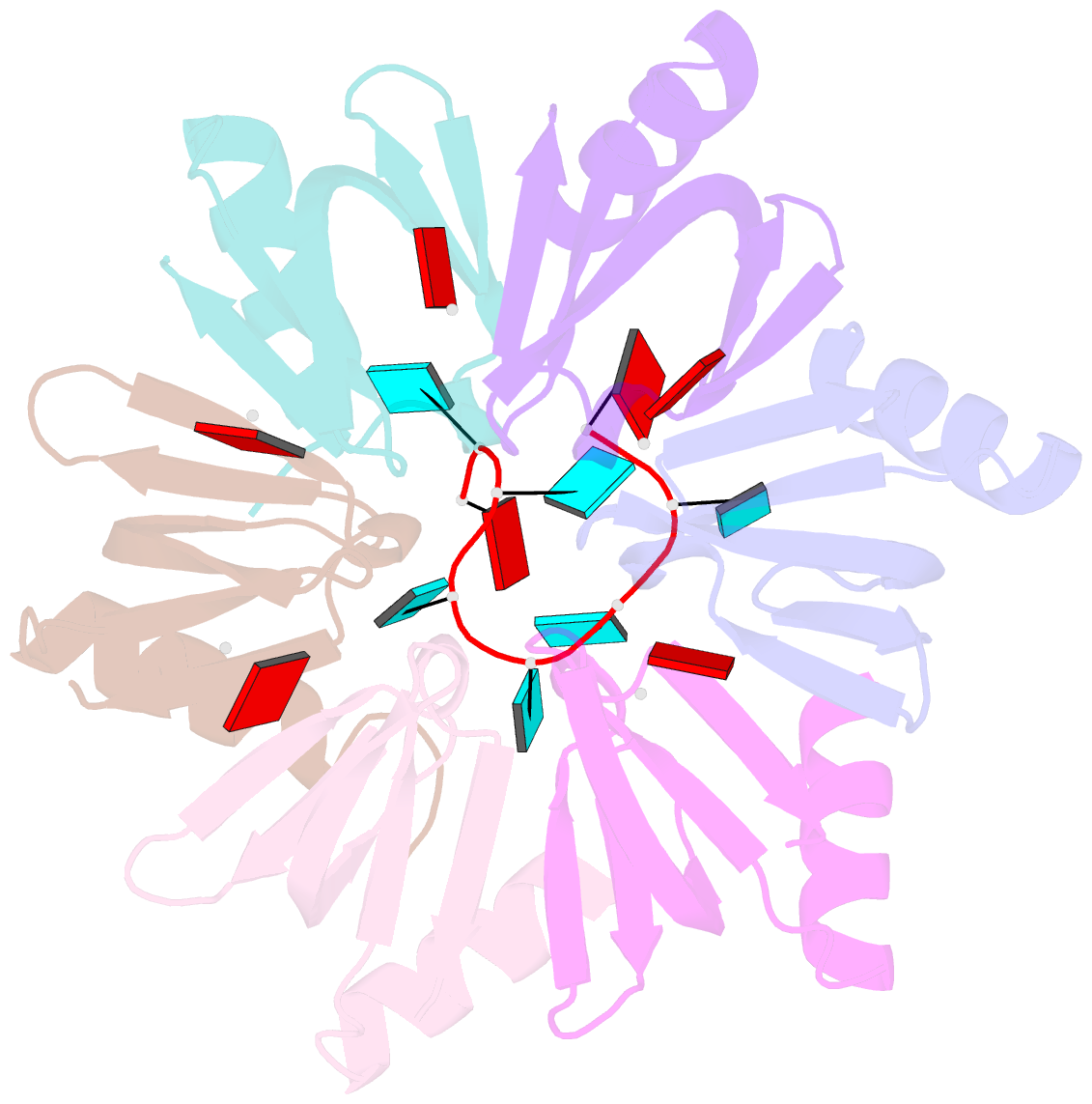
Summary information and primary citation
- PDB-id
- 3rer; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- chaperone-RNA
- Method
- X-ray (1.7 Å)
- Summary
- Crystal structure of e. coli hfq in complex with au6a RNA and adp
- Reference
- Wang W, Wang L, Zou Y, Zhang J, Gong Q, Wu J, Shi Y (2011): "Cooperation of Escherichia coli Hfq hexamers in DsrA binding." Genes Dev., 25, 2106-2117. doi: 10.1101/gad.16746011.
- Abstract
- Hfq is a bacterial post-transcriptional regulator. It facilitates base-pairing between sRNA and target mRNA. Hfq mediates DsrA-dependent translational activation of rpoS mRNA at low temperatures. rpoS encodes the stationary-phase σ factor σ(S), which is the central regulator in general stress response. However, structural information on Hfq-DsrA interaction is not yet available. Although Hfq is reported to hydrolyze ATP, the ATP-binding site is still unknown. Here, we report a ternary crystal complex structure of Escherichia coli Hfq bound to a major Hfq recognition region on DsrA (AU(6)A) together with ADP, and a crystal complex structure of Hfq bound to ADP. AU(6)A binds to the proximal and distal sides of two Hfq hexamers. ADP binds to a purine-selective site on the distal side and contacts conserved arginine or glutamine residues on the proximal side of another hexamer. This binding mode is different from previously postulated. The cooperation of two different Hfq hexamers upon nucleic acid binding in solution is verified by fluorescence polarization and solution nuclear magnetic resonance (NMR) experiments using fragments of Hfq and DsrA. Fluorescence resonance energy transfer conducted with full-length Hfq and DsrA also supports cooperation of Hfq hexamers upon DsrA binding. The implications of Hfq hexamer cooperation have been discussed.