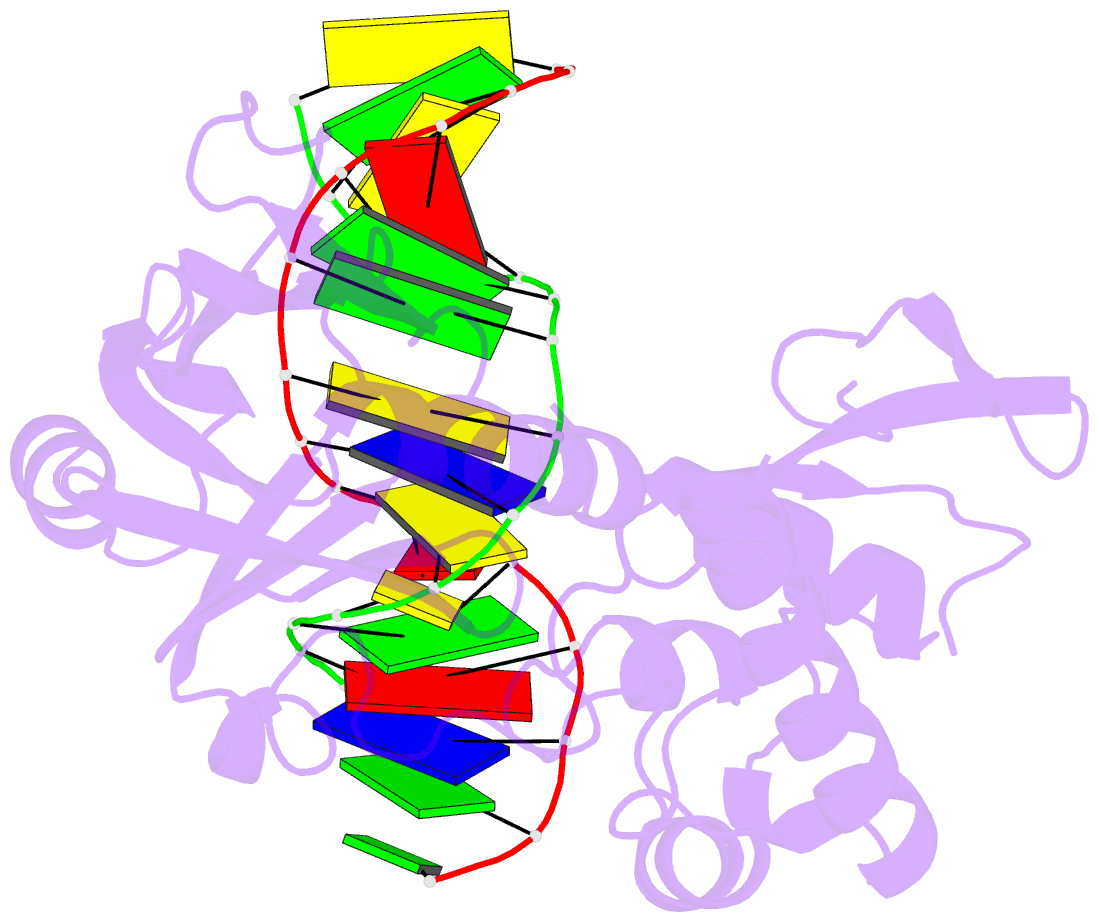
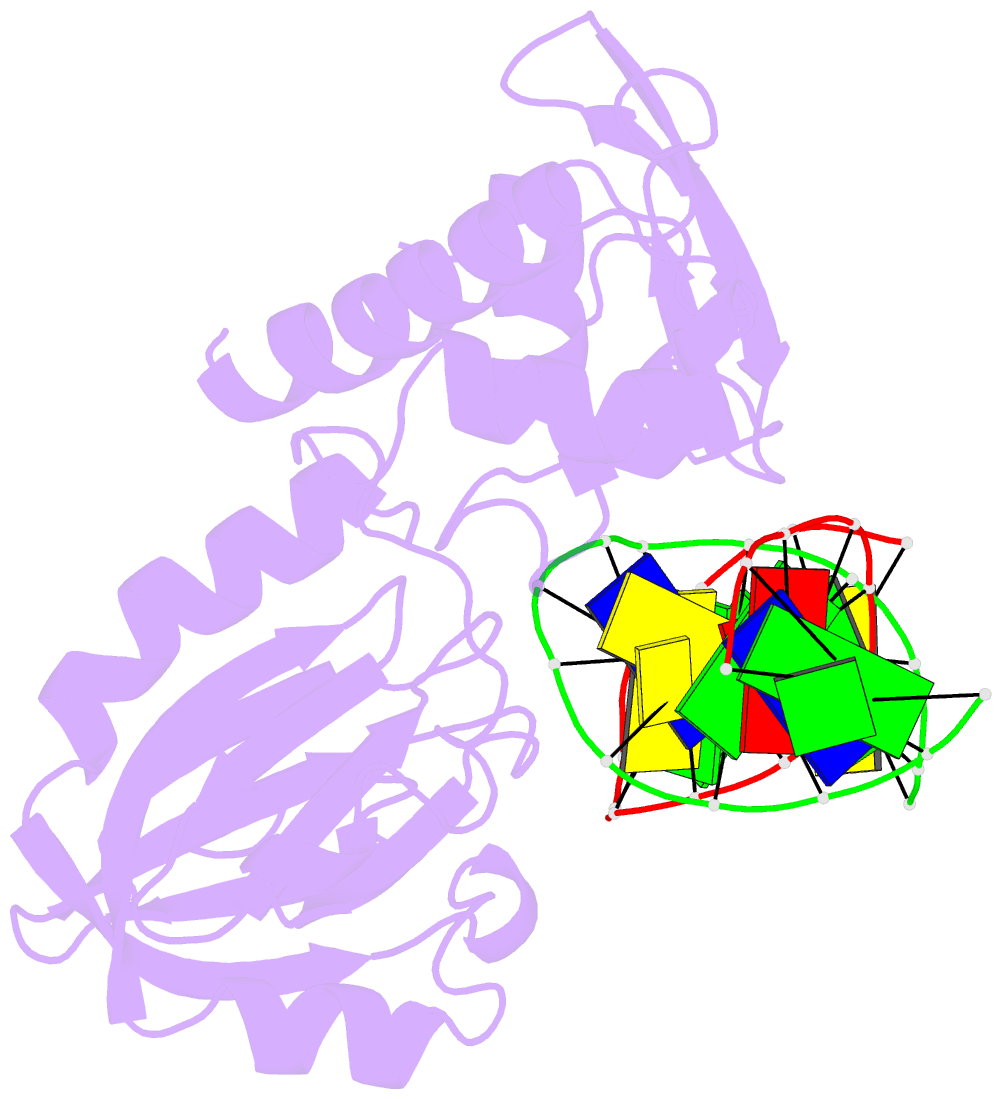
Summary information and primary citation
- PDB-id
- 3sas; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.05 Å)
- Summary
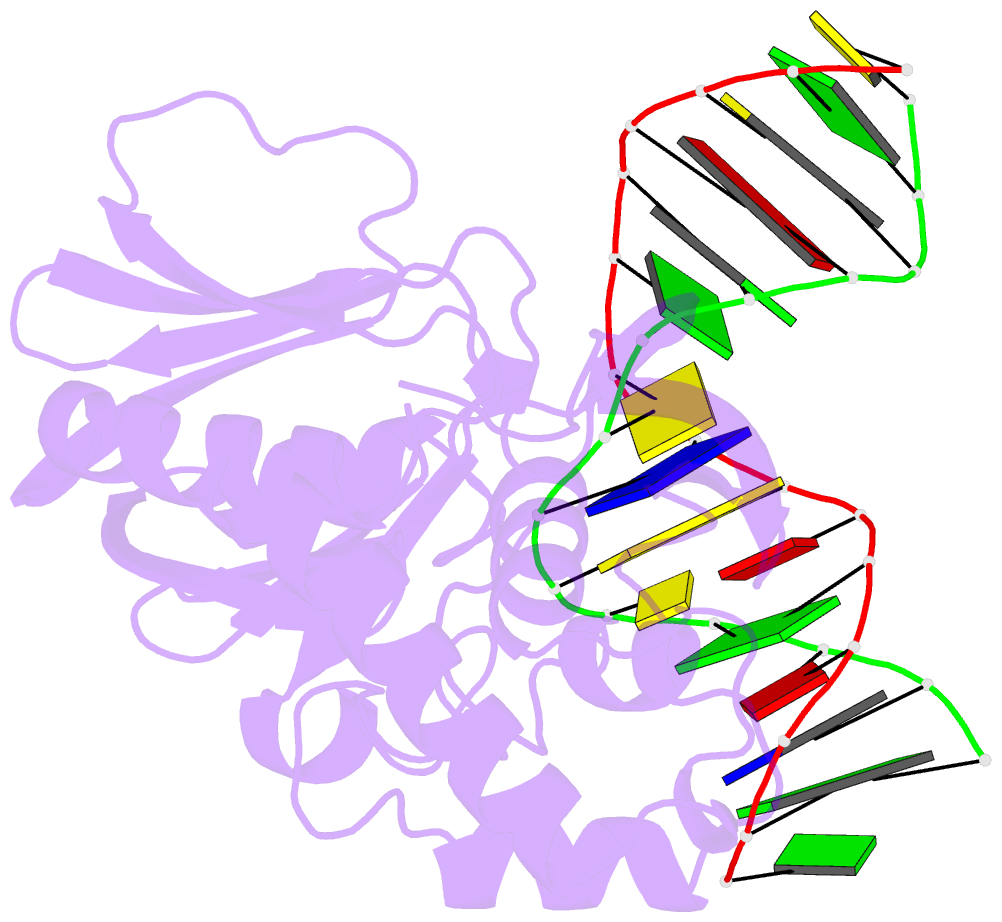
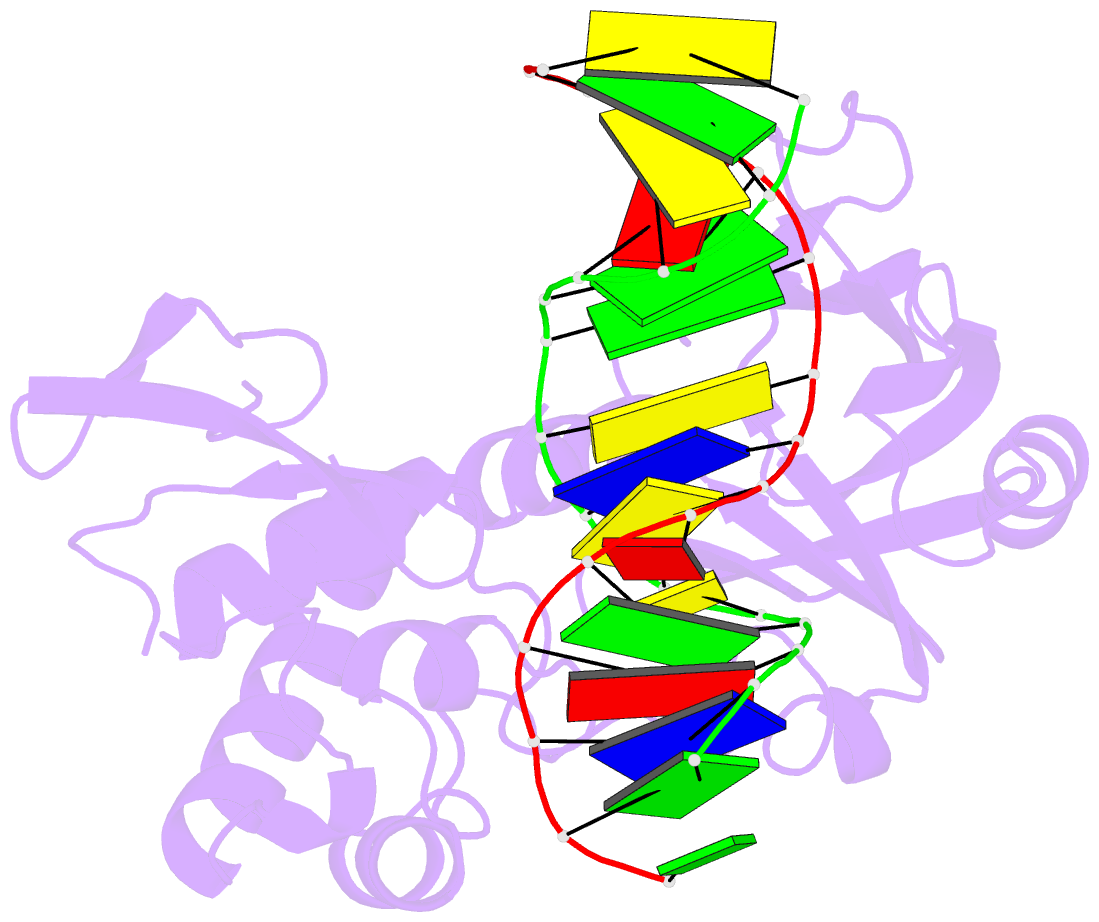
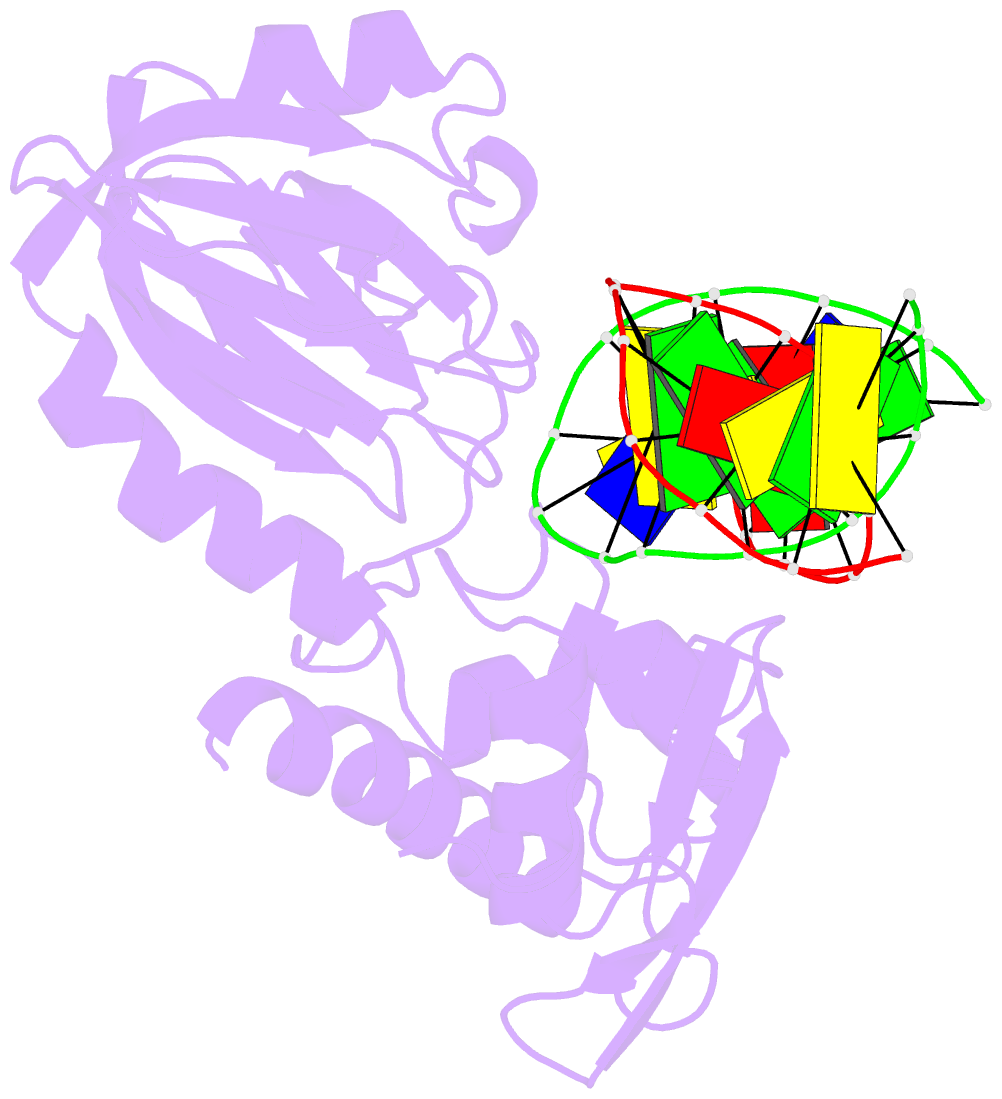
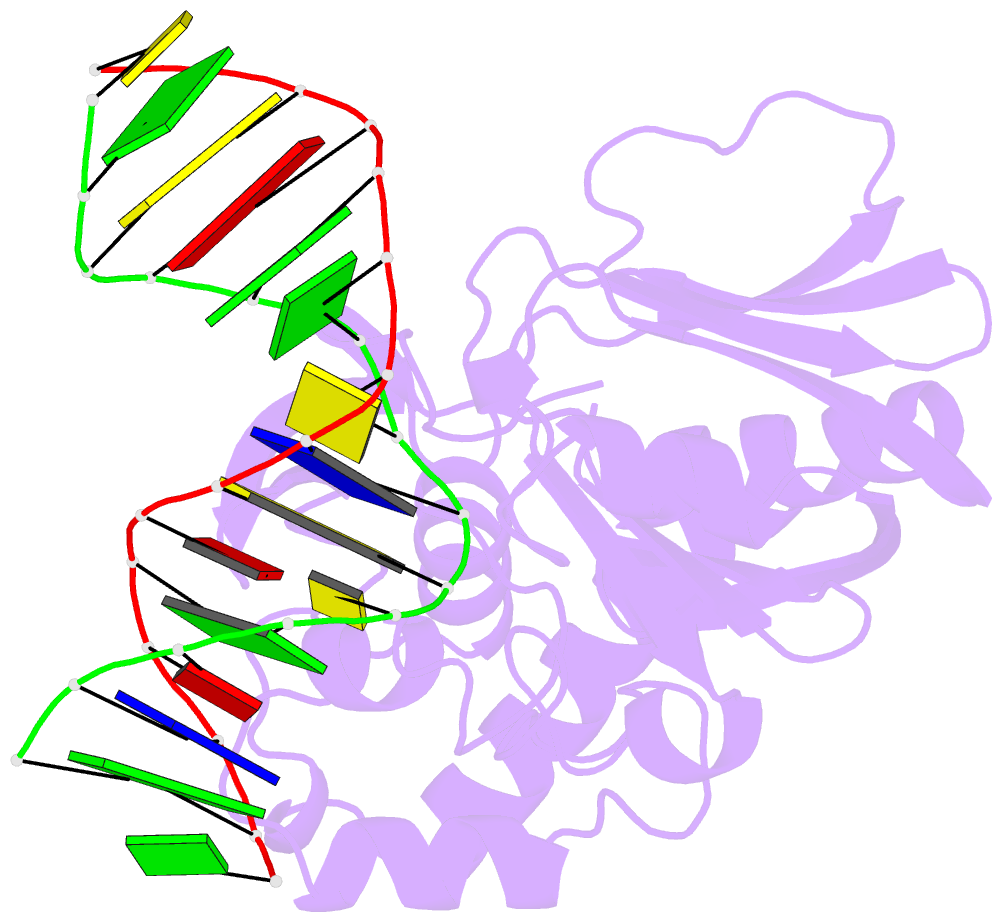
- Mutm slanted complex 4 with r112a mutation
- Reference
- Qi Y, Nam K, Spong MC, Banerjee A, Sung RJ, Zhang M, Karplus M, Verdine GL (2012): "Strandwise translocation of a DNA glycosylase on undamaged DNA." Proc.Natl.Acad.Sci.USA, 109, 1086-1091. doi: 10.1073/pnas.1111237108.
- Abstract
- Base excision repair of genotoxic nucleobase lesions in the genome is critically dependent upon the ability of DNA glycosylases to locate rare sites of damage embedded in a vast excess of undamaged DNA, using only thermal energy to fuel the search process. Considerable interest surrounds the question of how DNA glycosylases translocate efficiently along DNA while maintaining their vigilance for target damaged sites. Here, we report the observation of strandwise translocation of 8-oxoguanine DNA glycosylase, MutM, along undamaged DNA. In these complexes, the protein is observed to translocate by one nucleotide on one strand while remaining untranslocated on the complementary strand. We further report that alterations of single base-pairs or a single amino acid substitution (R112A) can induce strandwise translocation. Molecular dynamics simulations confirm that MutM can translocate along DNA in a strandwise fashion. These observations reveal a previously unobserved mode of movement for a DNA-binding protein along the surface of DNA.