Summary information and primary citation
- PDB-id
- 3snp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- lyase-RNA
- Method
- X-ray (2.8 Å)
- Summary
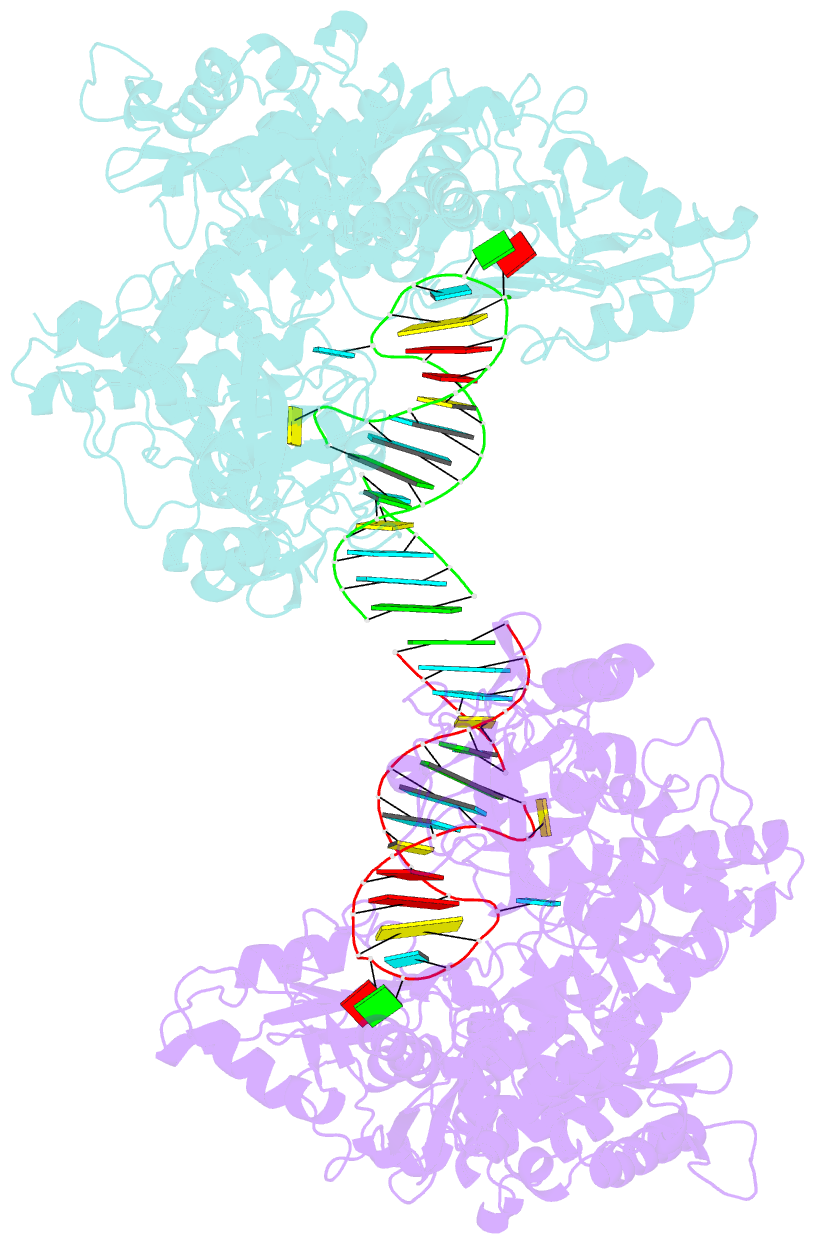
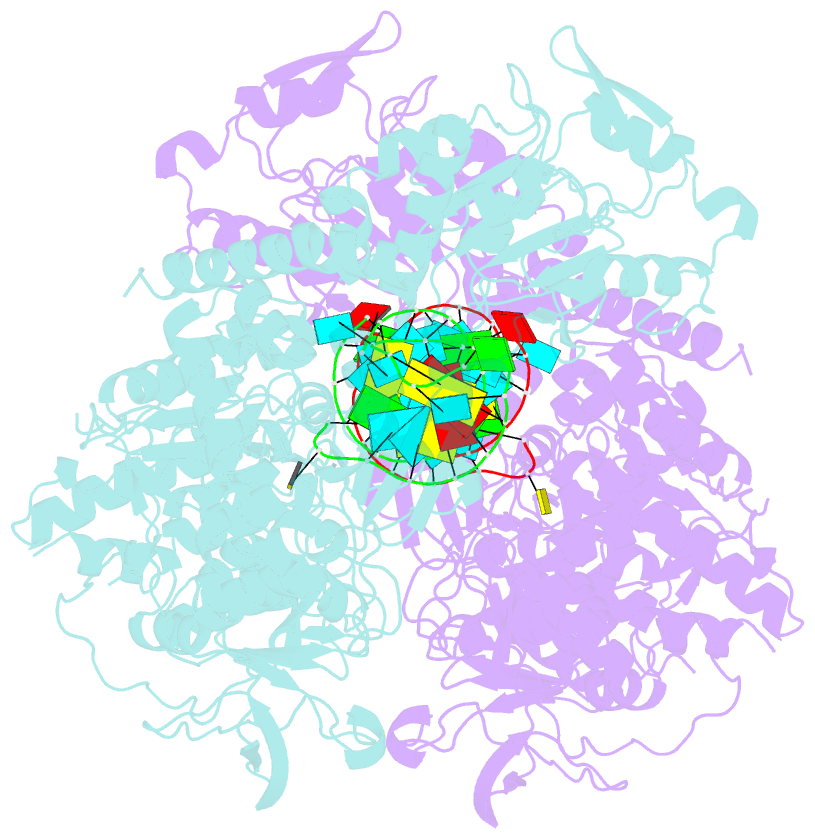
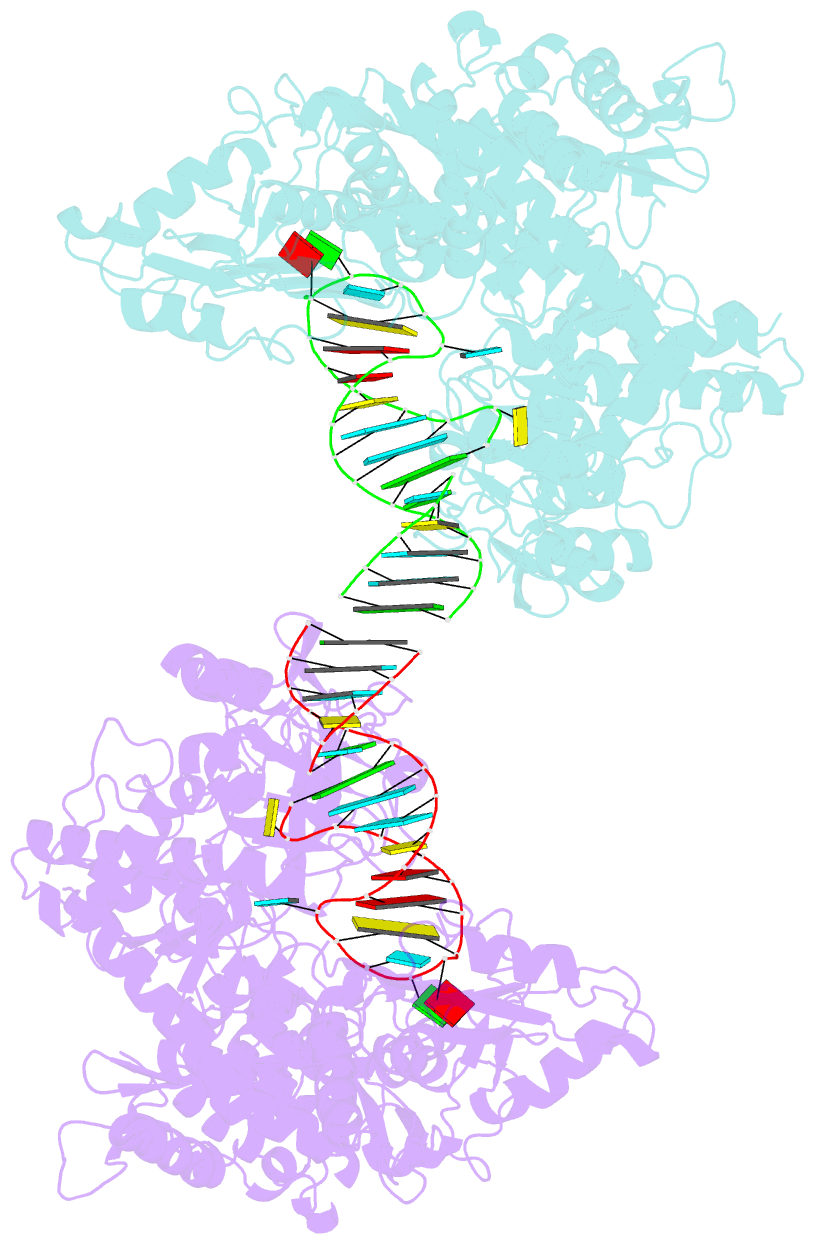
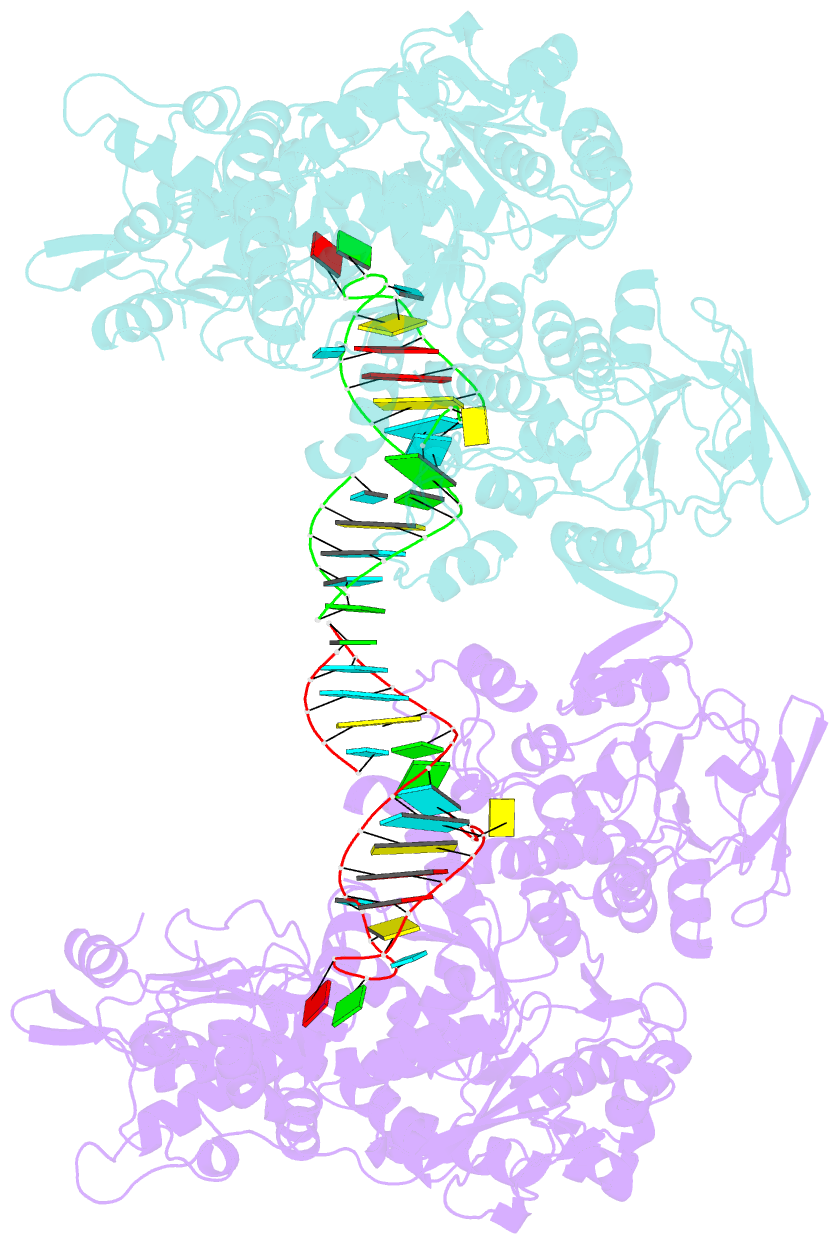
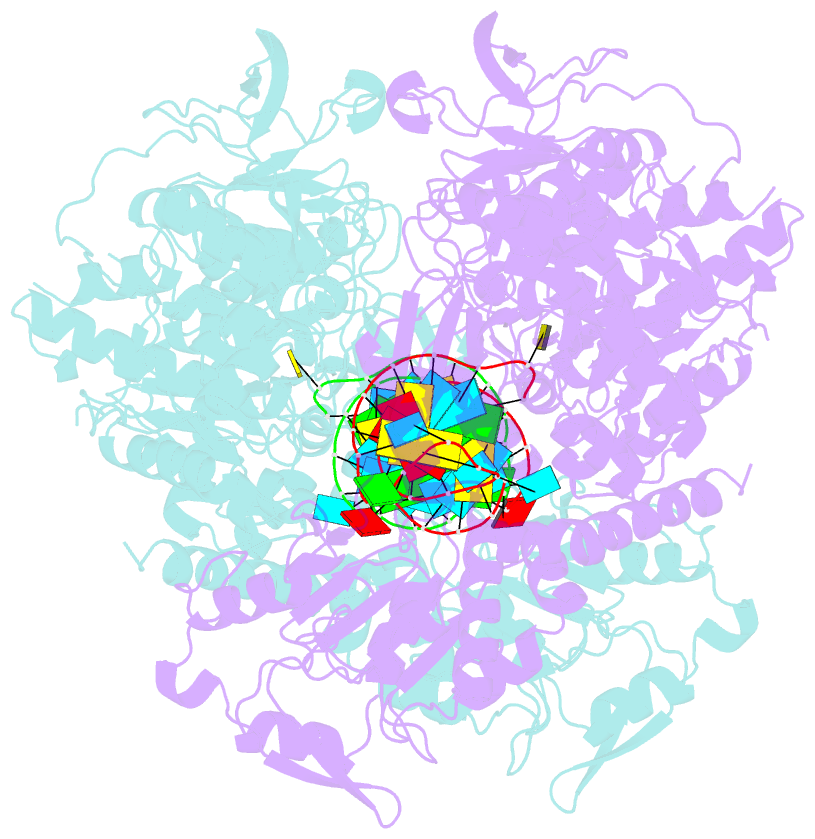
- Crystal structure analysis of iron regulatory protein 1 in complex with ferritin h ire RNA
- Reference
- Walden WE, Selezneva AI, Dupuy J, Volbeda A, Fontecilla-Camps JC, Theil EC, Volz K (2006): "Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA." Science, 314, 1903-1908. doi: 10.1126/science.1133116.
- Abstract
- Iron regulatory protein 1 (IRP1) binds iron-responsive elements (IREs) in messenger RNAs (mRNAs), to repress translation or degradation, or binds an iron-sulfur cluster, to become a cytosolic aconitase enzyme. The 2.8 angstrom resolution crystal structure of the IRP1:ferritin H IRE complex shows an open protein conformation compared with that of cytosolic aconitase. The extended, L-shaped IRP1 molecule embraces the IRE stem-loop through interactions at two sites separated by approximately 30 angstroms, each involving about a dozen protein:RNA bonds. Extensive conformational changes related to binding the IRE or an iron-sulfur cluster explain the alternate functions of IRP1 as an mRNA regulator or enzyme.