Summary information and primary citation
- PDB-id
- 3t5n; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-RNA
- Method
- X-ray (1.787 Å)
- Summary
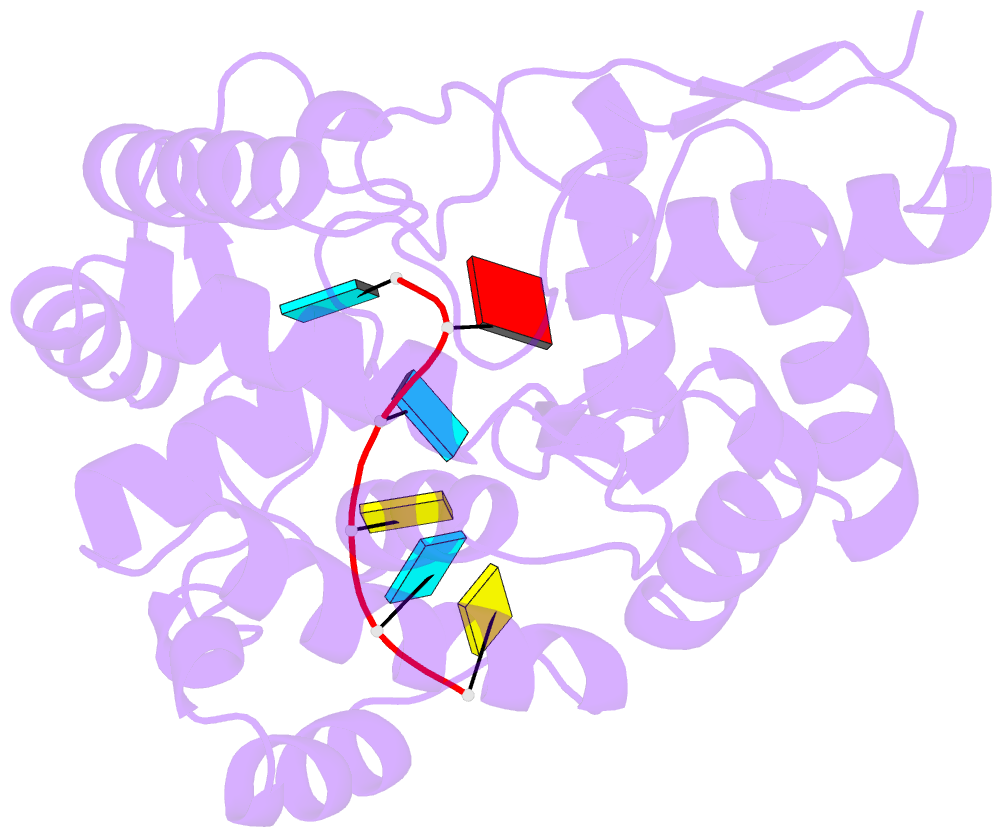
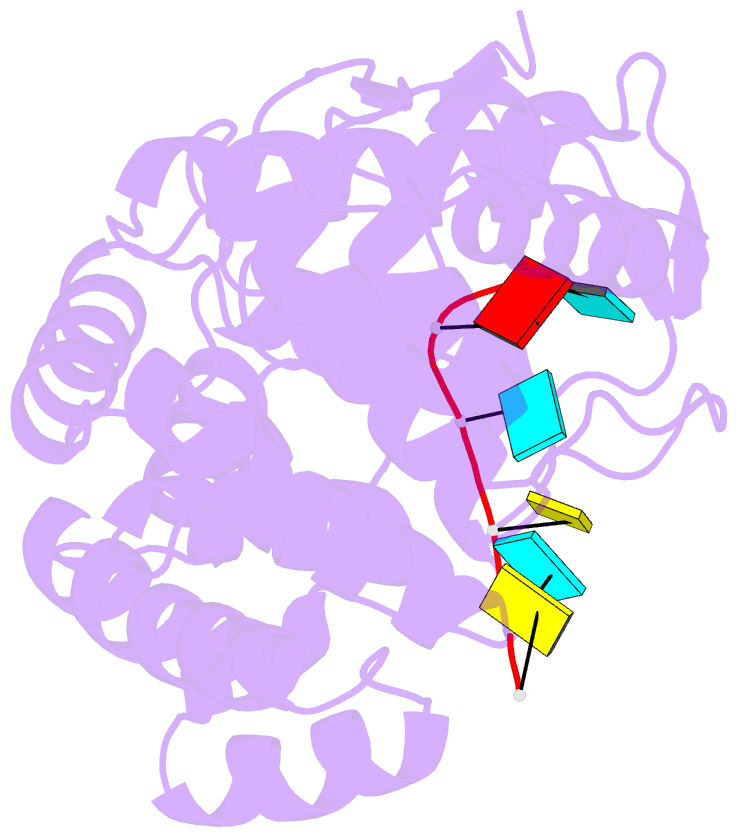
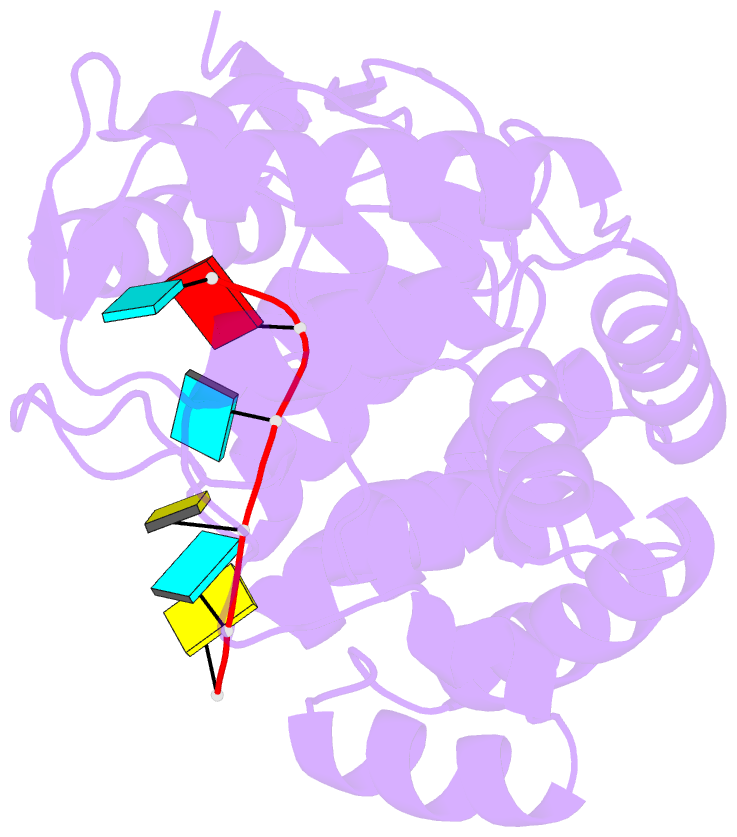
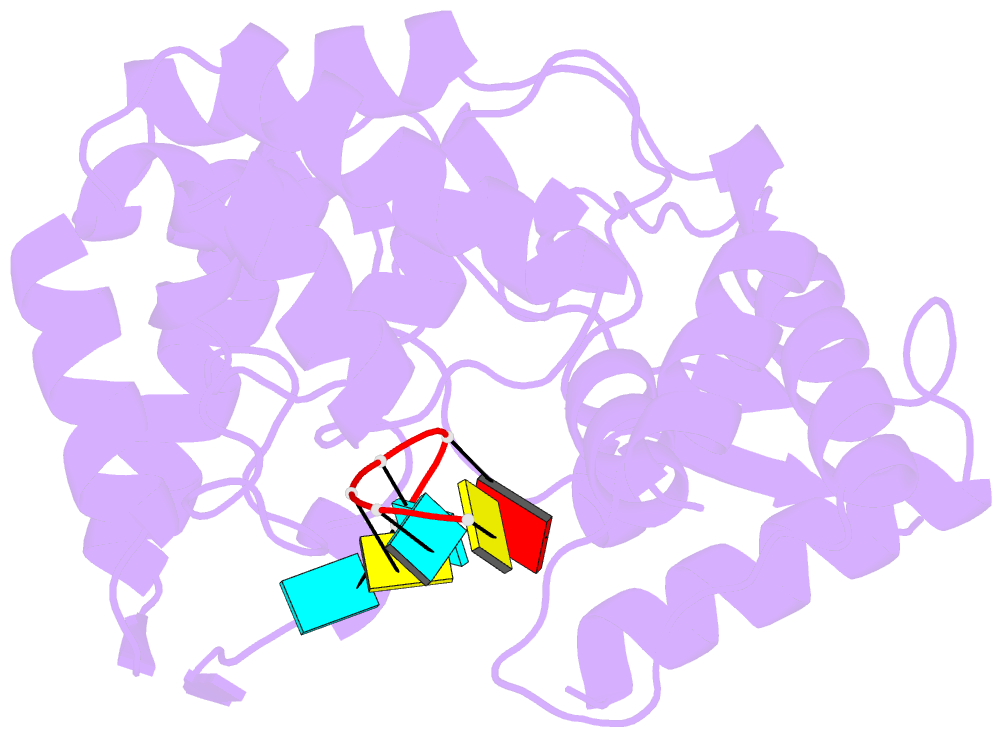
- 1.8a crystal structure of lassa virus nucleoprotein in complex with ssrna
- Reference
- Hastie KM, Liu T, Li S, King LB, Ngo N, Zandonatti MA, Woods VL, de la Torre JC, Saphire EO (2011): "Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding." Proc.Natl.Acad.Sci.USA, 108, 19365-19370. doi: 10.1073/pnas.1108515108.
- Abstract
- Arenaviruses cause disease in industrialized and developing nations alike. Among them, the hemorrhagic fever virus Lassa is responsible for ~300,000-500,000 infections/y in Western Africa. The arenavirus nucleoprotein (NP) forms the protein scaffold of the genomic ribonucleoprotein complexes and is critical for transcription and replication of the viral genome. Here, we present crystal structures of the RNA-binding domain of Lassa virus NP in complex with ssRNA. This structure shows, in contrast to the predicted model, that RNA binds in a deep, basic crevice located entirely within the N-terminal domain. Furthermore, the NP-ssRNA structures presented here, combined with hydrogen-deuterium exchange/MS and functional studies, suggest a gating mechanism by which NP opens to accept RNA. Directed mutagenesis and functional studies provide a unique look into how the arenavirus NPs bind to and protect the viral genome and also suggest the likely assembly by which viral ribonucleoprotein complexes are organized.