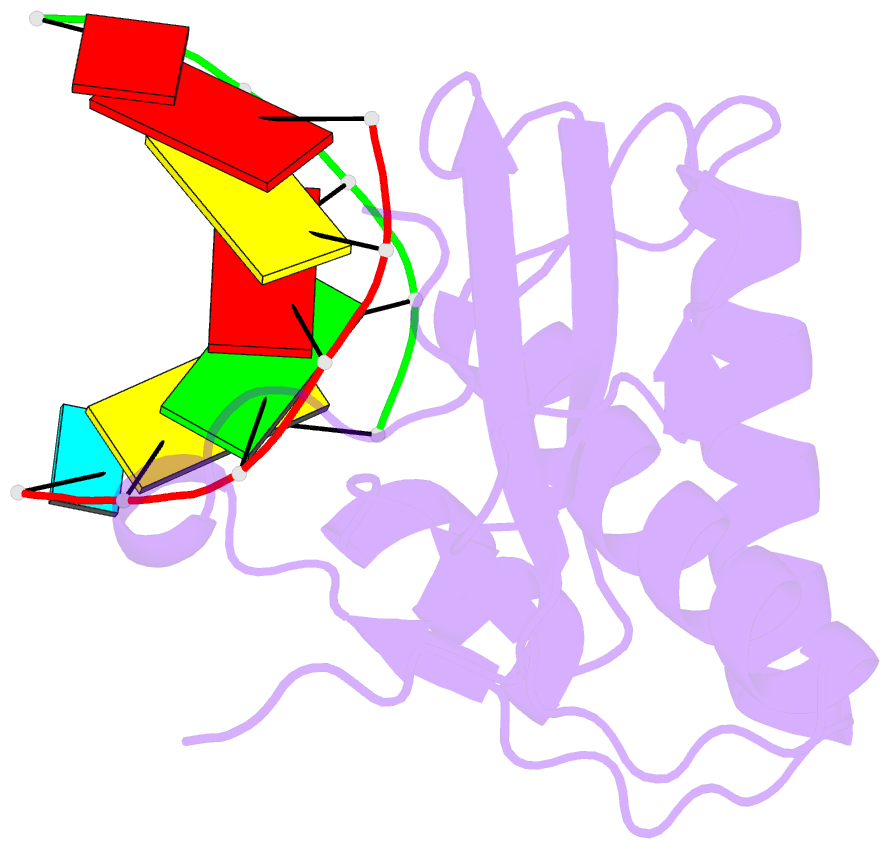
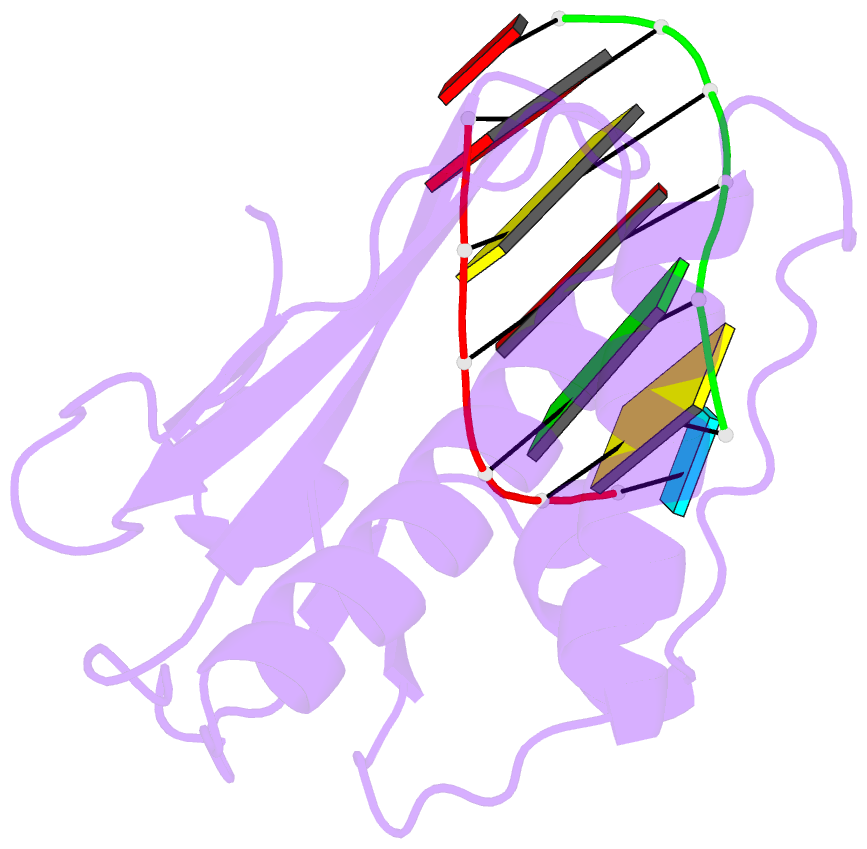
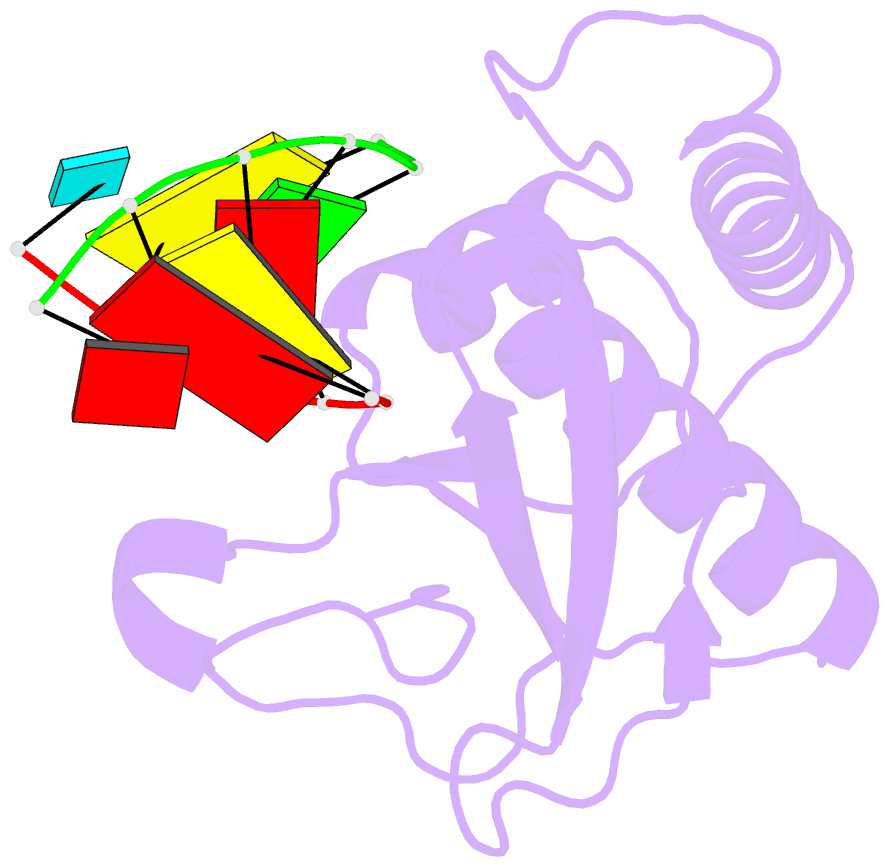
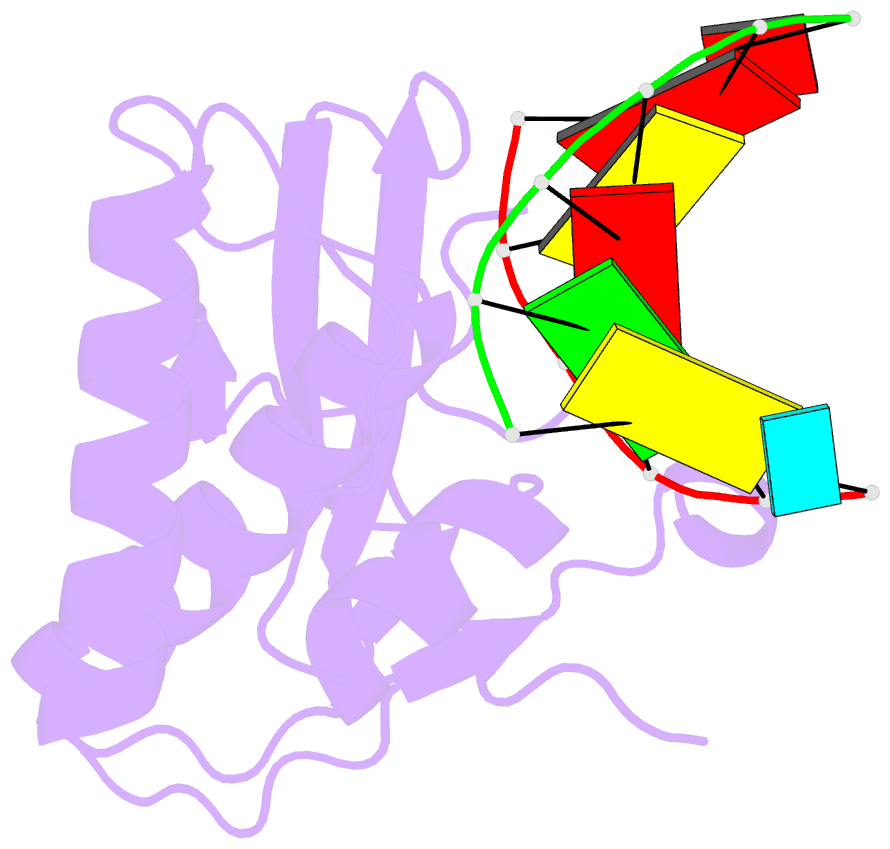
Summary information and primary citation
- PDB-id
- 3twh; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA-DNA
- Method
- X-ray (1.79 Å)
- Summary
- Selenium derivatized RNA-DNA hybrid in complex with rnase h catalytic domain d132n mutant
- Reference
- Abdur R, Gerlits OO, Gan J, Jiang J, Salon J, Kovalevsky AY, Chumanevich AA, Weber IT, Huang Z (2014): "Novel complex MAD phasing and RNase H structural insights using selenium oligonucleotides." Acta Crystallogr.,Sect.D, 70, 354-361. doi: 10.1107/S1399004713027922.
- Abstract
- The crystal structures of protein-nucleic acid complexes are commonly determined using selenium-derivatized proteins via MAD or SAD phasing. Here, the first protein-nucleic acid complex structure determined using selenium-derivatized nucleic acids is reported. The RNase H-RNA/DNA complex is used as an example to demonstrate the proof of principle. The high-resolution crystal structure indicates that this selenium replacement results in a local subtle unwinding of the RNA/DNA substrate duplex, thereby shifting the RNA scissile phosphate closer to the transition state of the enzyme-catalyzed reaction. It was also observed that the scissile phosphate forms a hydrogen bond to the water nucleophile and helps to position the water molecule in the structure. Consistently, it was discovered that the substitution of a single O atom by a Se atom in a guide DNA sequence can largely accelerate RNase H catalysis. These structural and catalytic studies shed new light on the guide-dependent RNA cleavage.