Summary information and primary citation
- PDB-id
- 3ulp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.1 Å)
- Summary
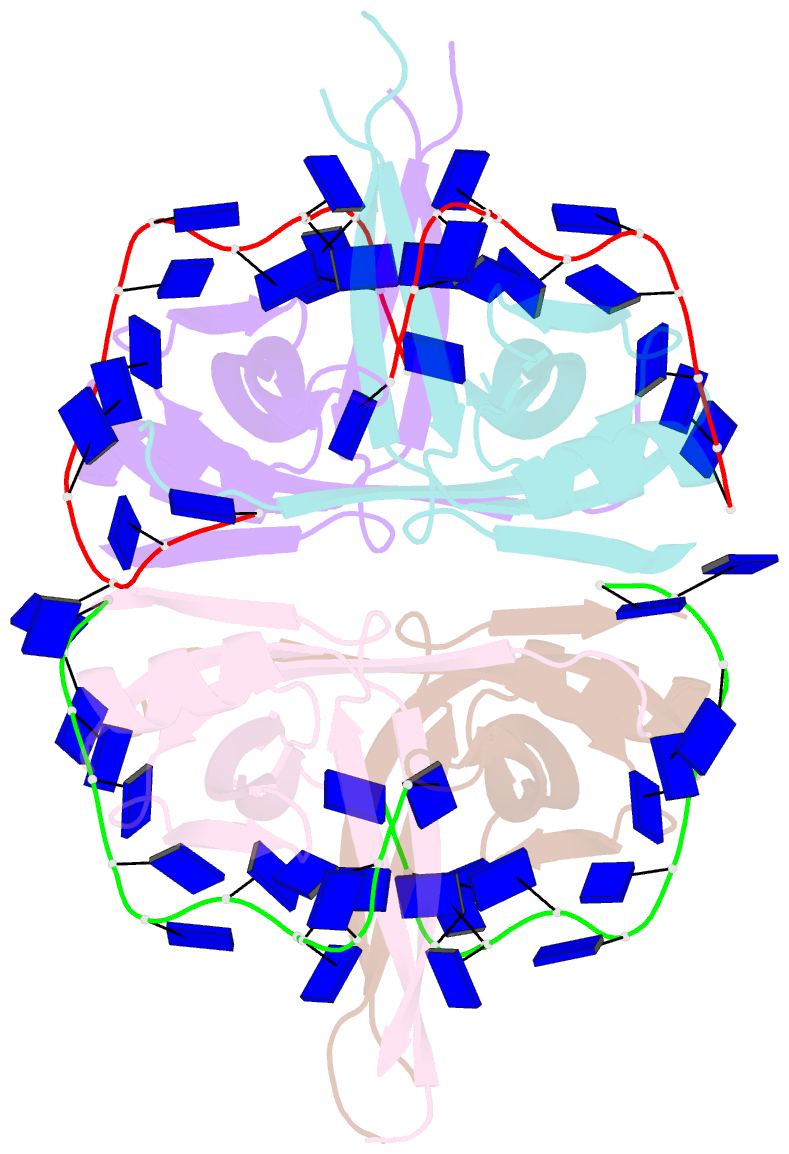
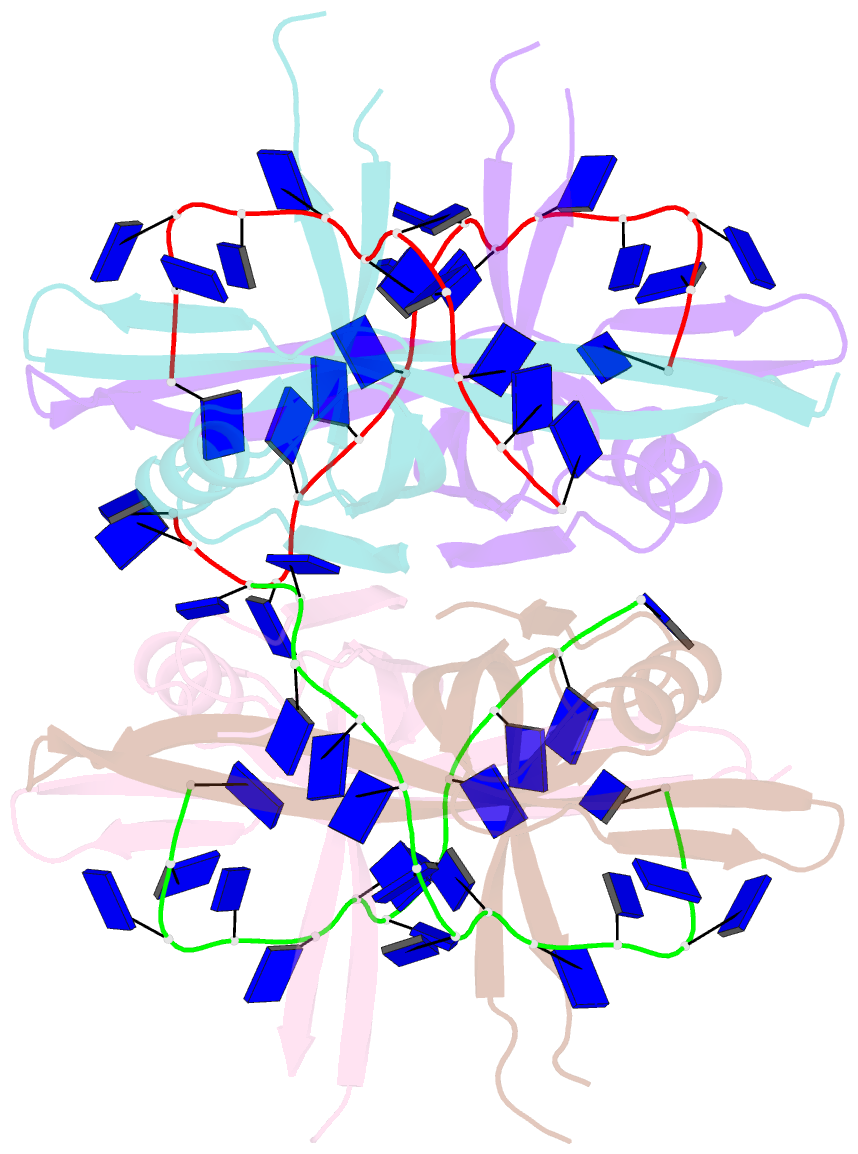
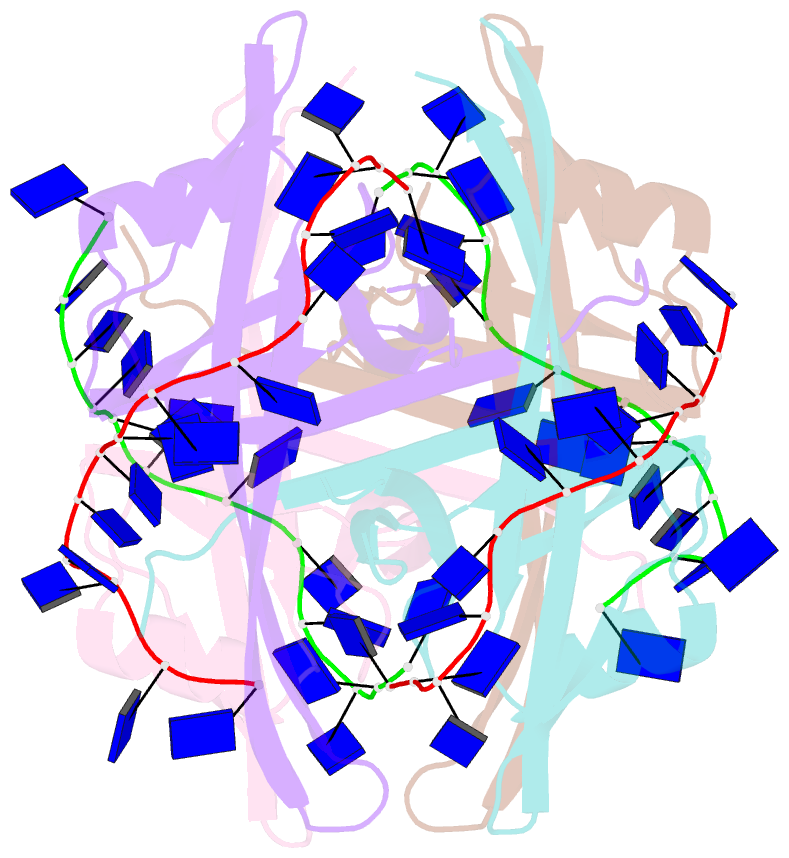
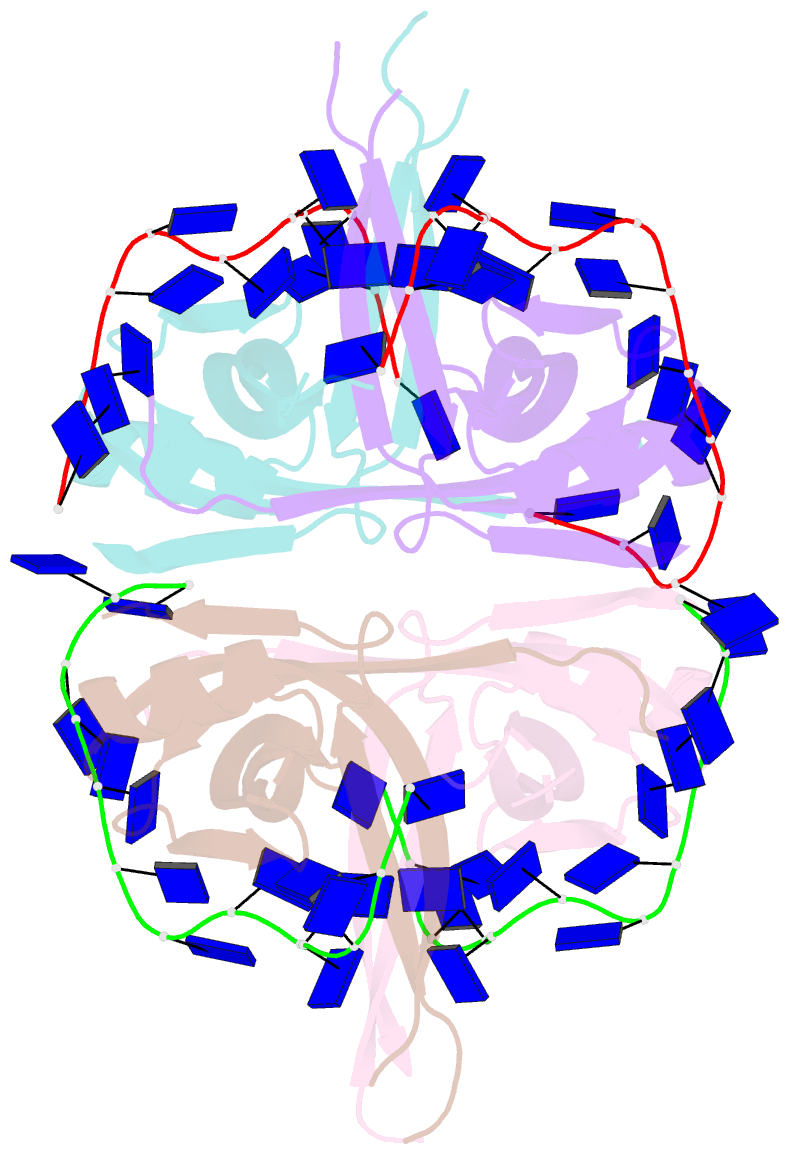
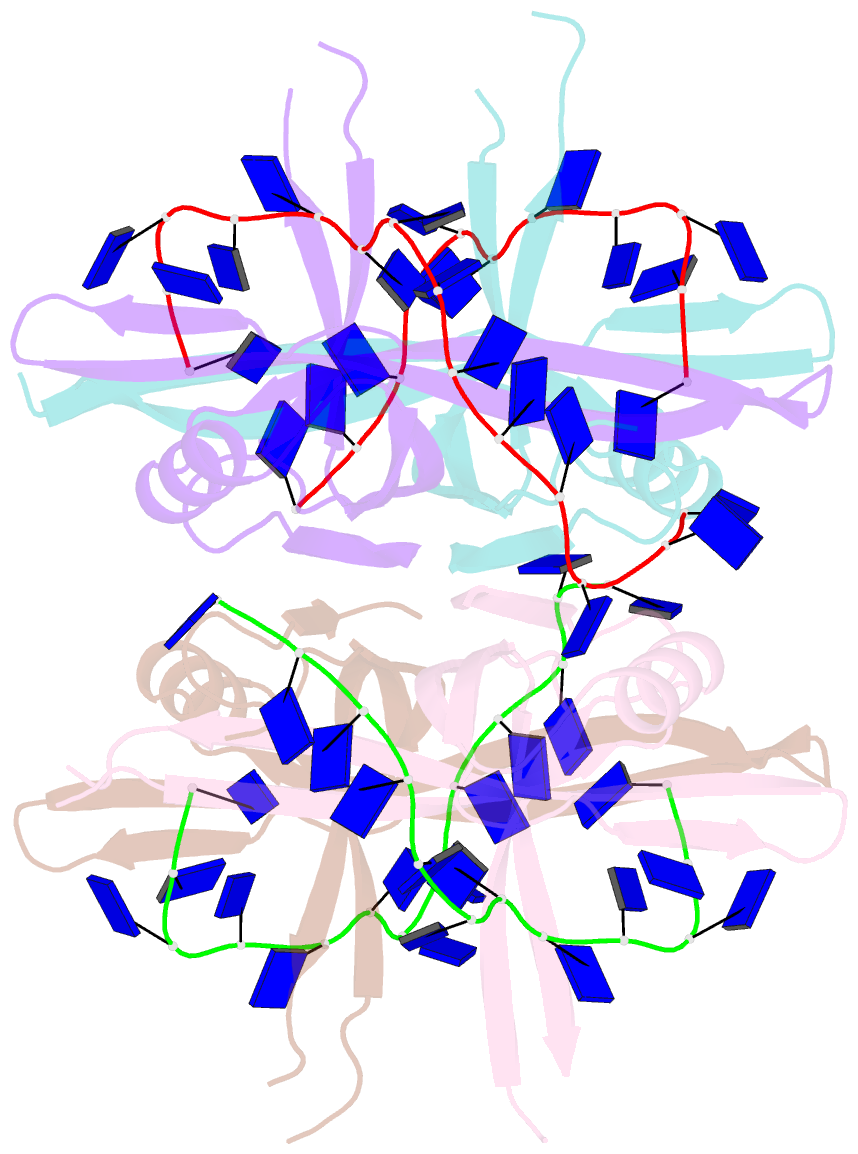
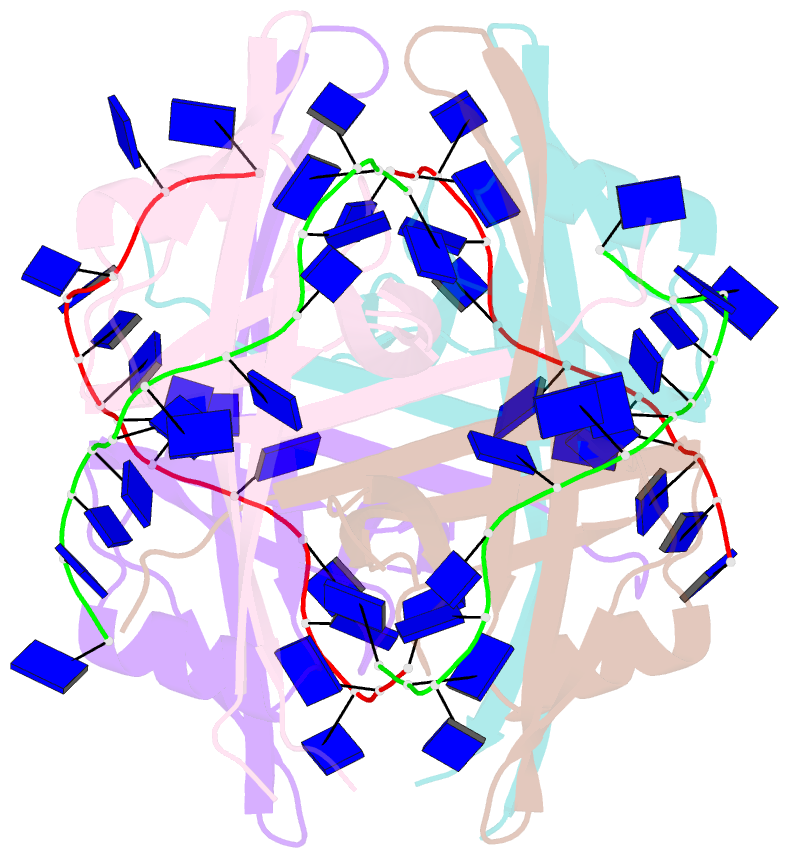
- Plasmodium falciparum ssb complex with ssDNA
- Reference
- Antony E, Weiland EA, Korolev S, Lohman TM (2012): "Plasmodium falciparum SSB Tetramer Wraps Single-Stranded DNA with Similar Topology but Opposite Polarity to E. coli SSB." J.Mol.Biol., 420, 269-283. doi: 10.1016/j.jmb.2012.04.021.
- Abstract
- Single-stranded DNA binding (SSB) proteins play central roles in genome maintenance in all organisms. Plasmodium falciparum, the causative agent of malaria, encodes an SSB protein that localizes to the apicoplast and likely functions in the replication and maintenance of its genome. P. falciparum SSB (Pf-SSB) shares a high degree of sequence homology with bacterial SSB proteins but differs in the composition of its C-terminus, which interacts with more than a dozen other proteins in Escherichia coli SSB (Ec-SSB). Using sedimentation methods, we show that Pf-SSB forms a stable homo-tetramer alone and when bound to single-stranded DNA (ssDNA). We also present a crystal structure at 2.1 Å resolution of the Pf-SSB tetramer bound to two (dT)(35) molecules. The Pf-SSB tetramer is structurally similar to the Ec-SSB tetramer, and ssDNA wraps completely around the tetramer with a "baseball seam" topology that is similar to Ec-SSB in its "65 binding mode". However, the polarity of the ssDNA wrapping around Pf-SSB is opposite to that observed for Ec-SSB. The interactions between the bases in the DNA and the amino acid side chains also differ from those observed in the Ec-SSB-DNA structure, suggesting that other differences may exist in the DNA binding properties of these structurally similar proteins.