Summary information and primary citation
- PDB-id
- 3uob; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.011 Å)
- Summary
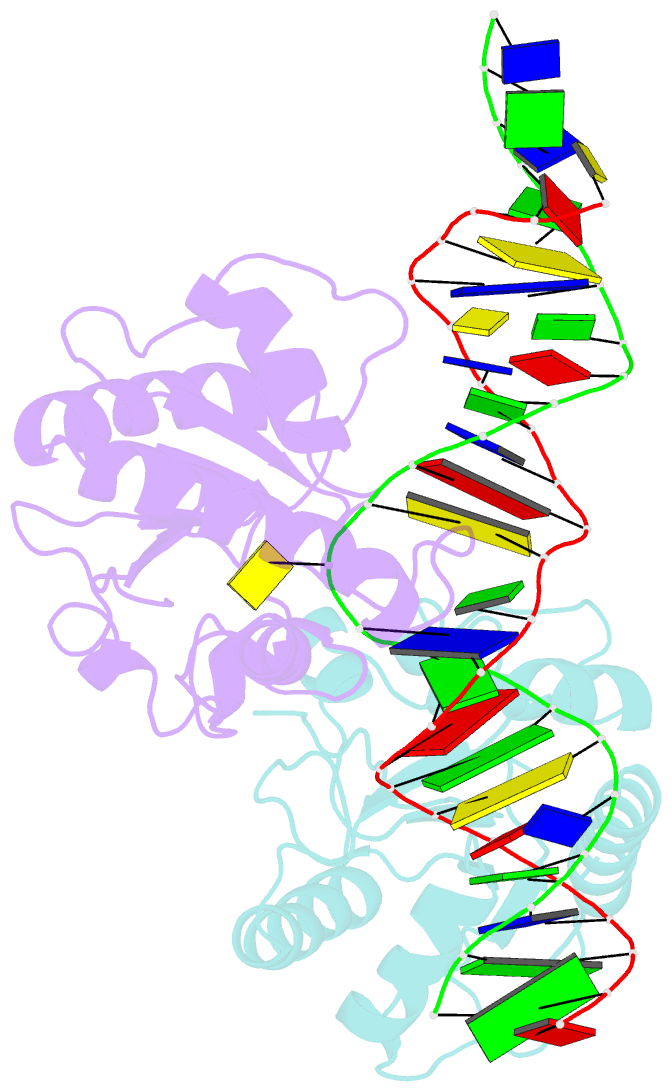
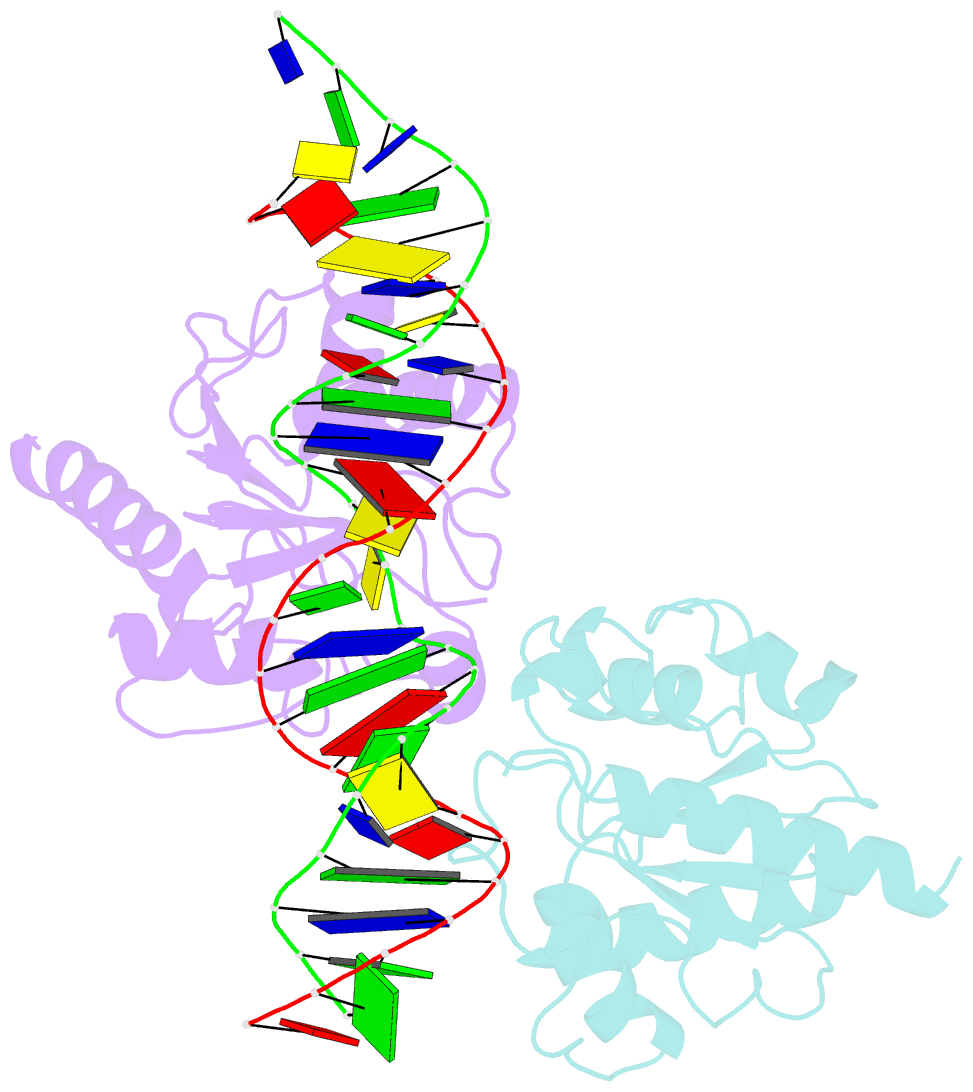
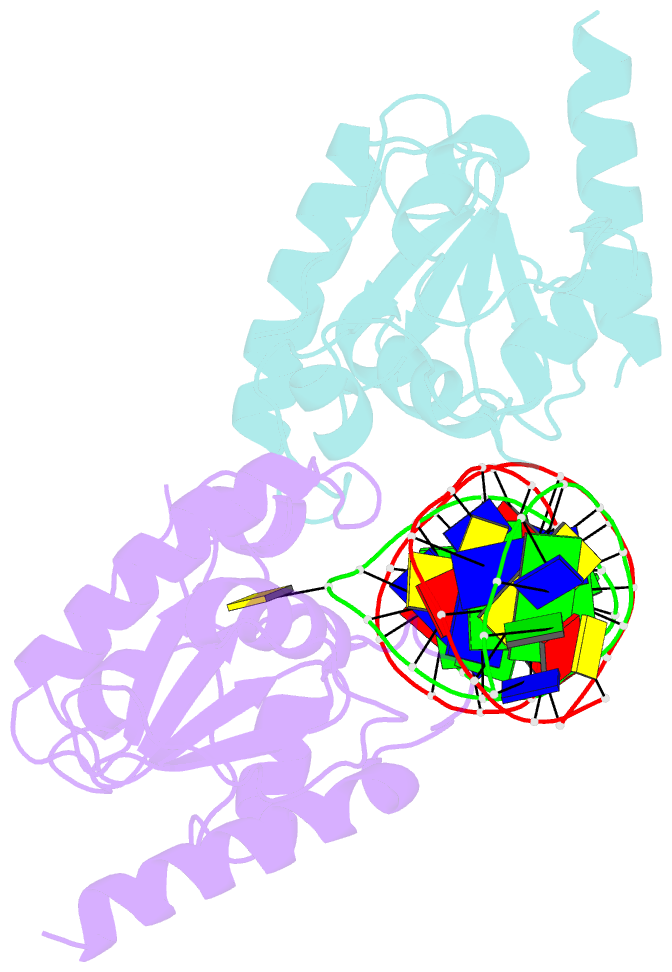
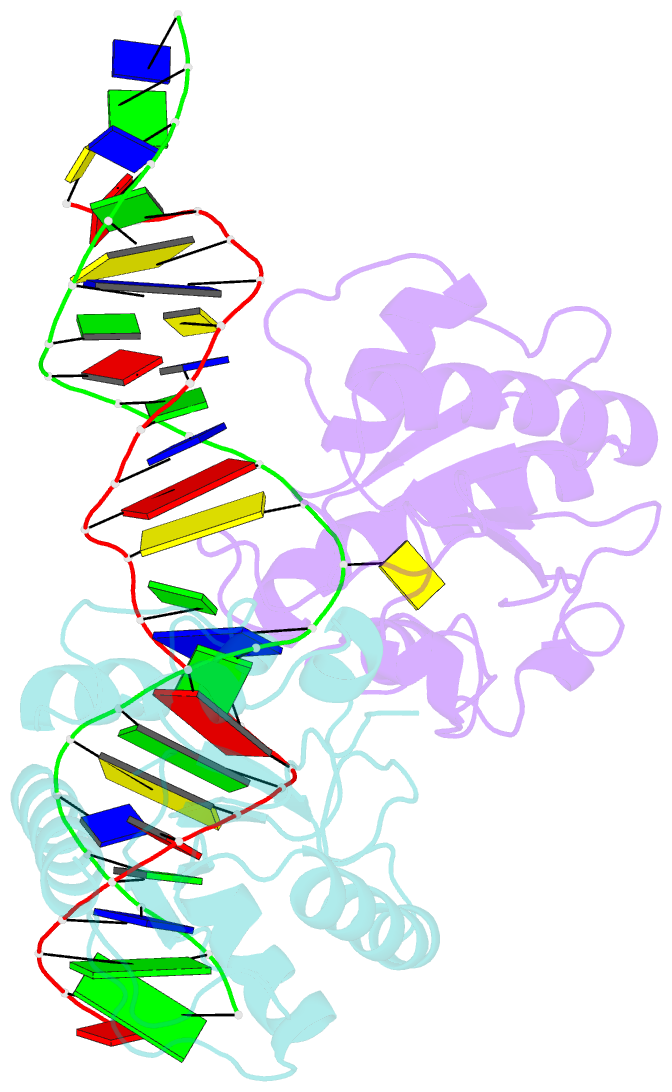
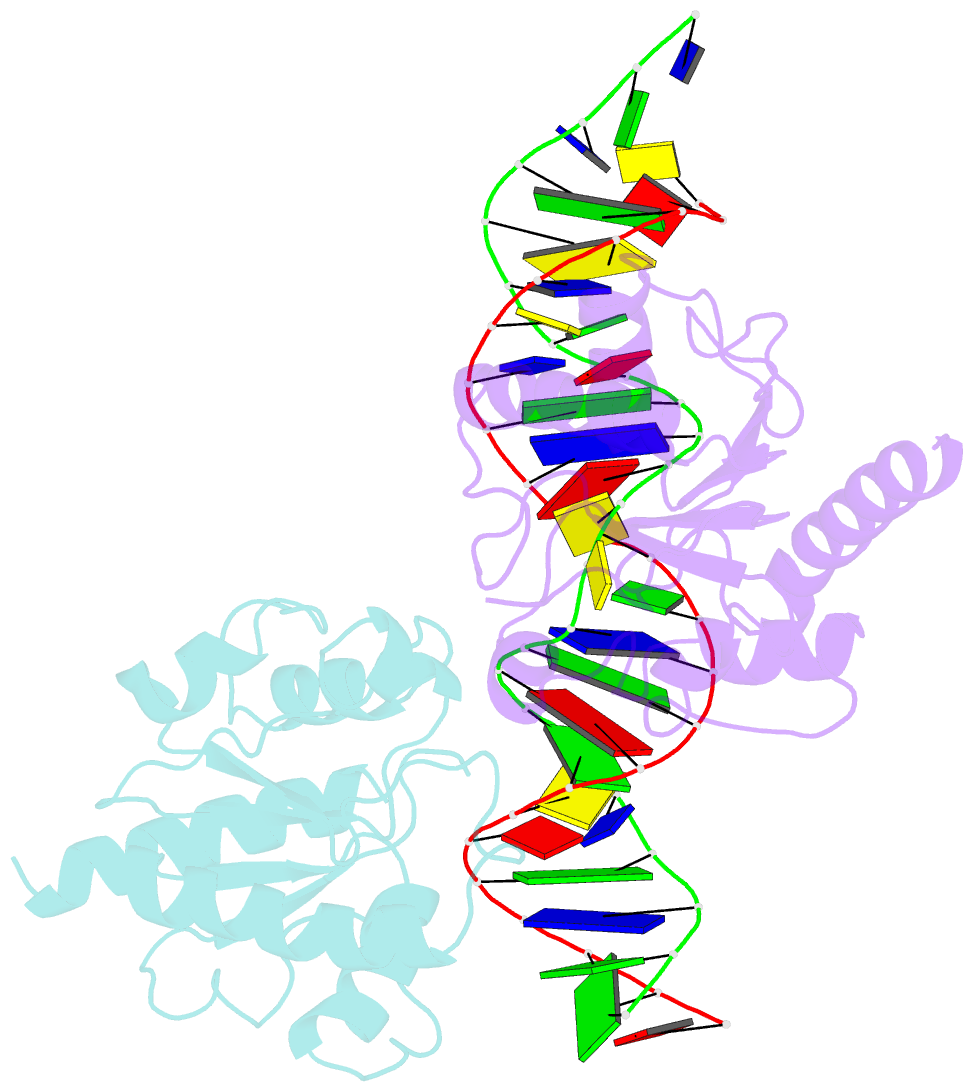
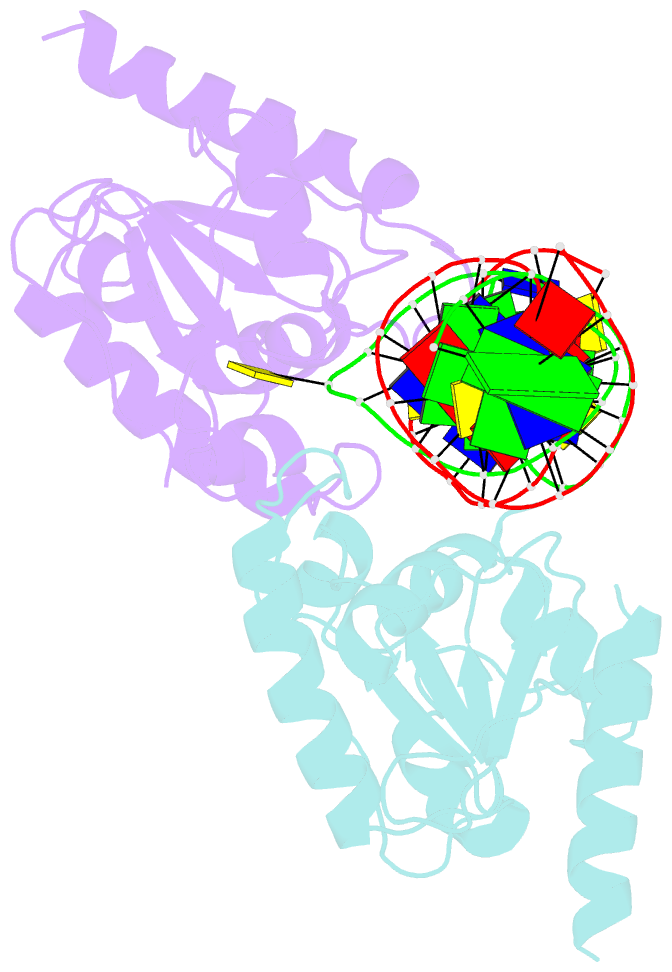
- Crystal structure of human thymine DNA glycosylase bound to substrate analog 2'-deoxy-2'-beta-fluoro-cytidine
- Reference
- Zhang L, Lu X, Lu J, Liang H, Dai Q, Xu GL, Luo C, Jiang H, He C (2012): "Thymine DNA glycosylase specifically recognizes 5-carboxylcytosine-modified DNA." Nat.Chem.Biol., 8, 328-330. doi: 10.1038/nchembio.914.
- Abstract
- Human thymine DNA glycosylase (hTDG) efficiently excises 5-carboxylcytosine (5caC), a key oxidation product of 5-methylcytosine in genomic DNA, in a recently discovered cytosine demethylation pathway. We present here the crystal structures of the hTDG catalytic domain in complex with duplex DNA containing either 5caC or a fluorinated analog. These structures, together with biochemical and computational analyses, reveal that 5caC is specifically recognized in the active site of hTDG, supporting the role of TDG in mammalian 5-methylcytosine demethylation.