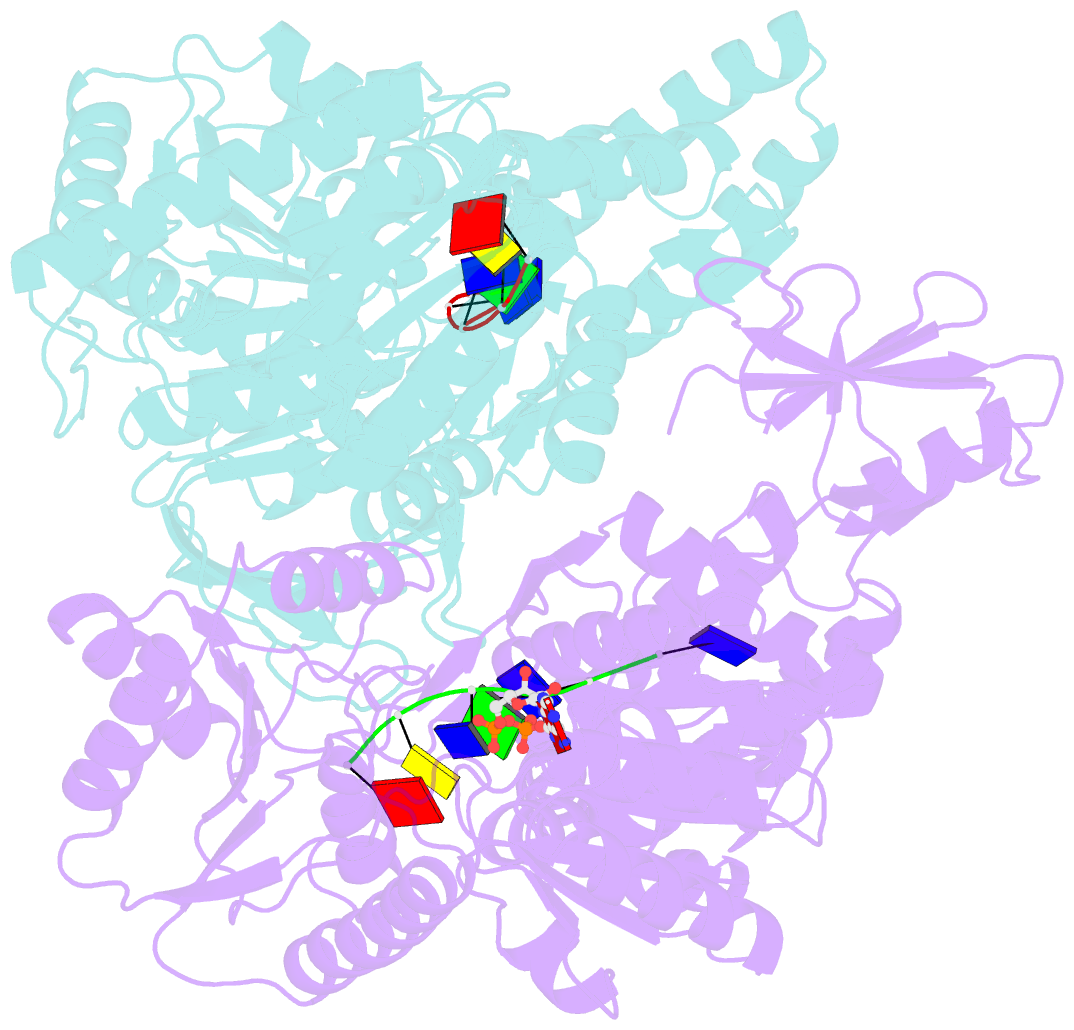
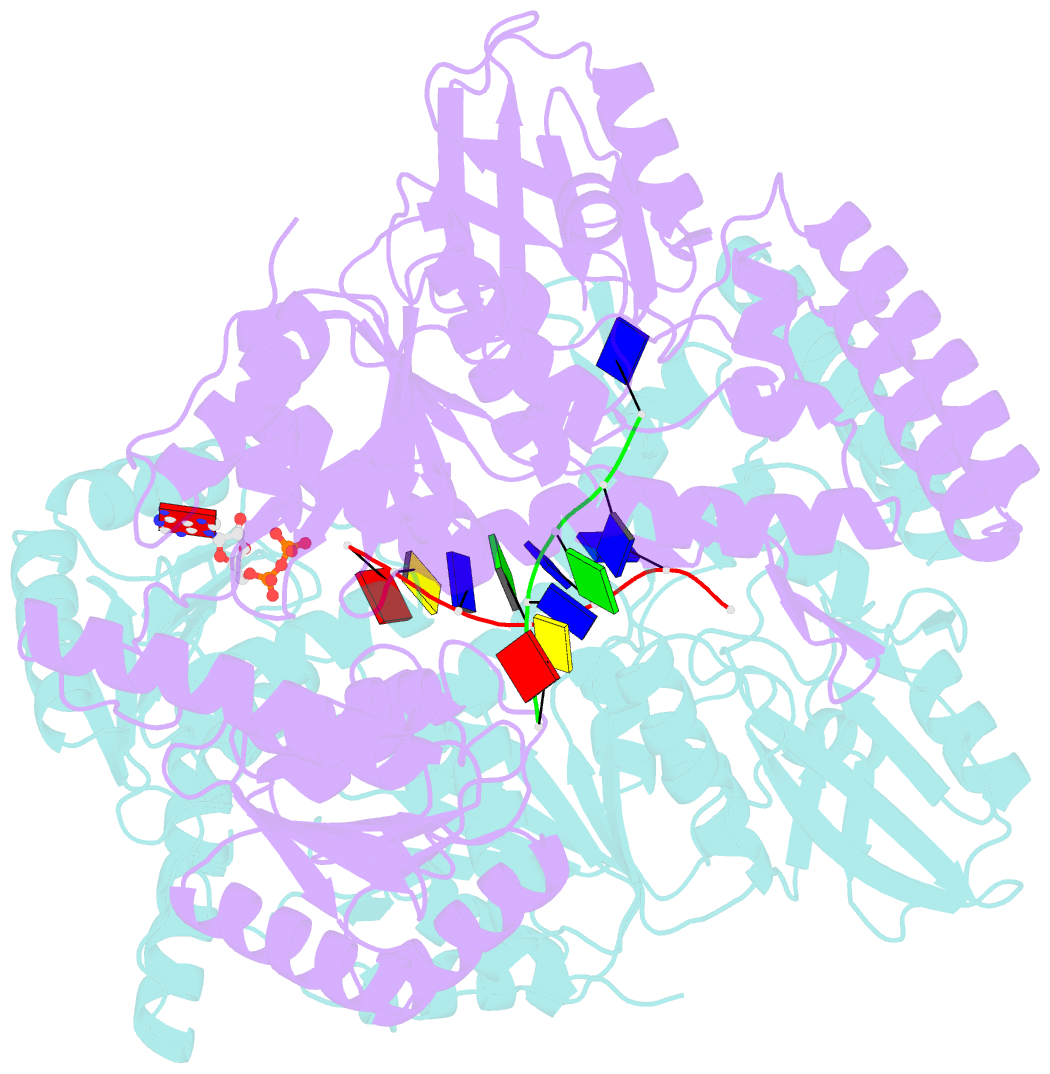
Summary information and primary citation
- PDB-id
- 3v4r; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.25 Å)
- Summary
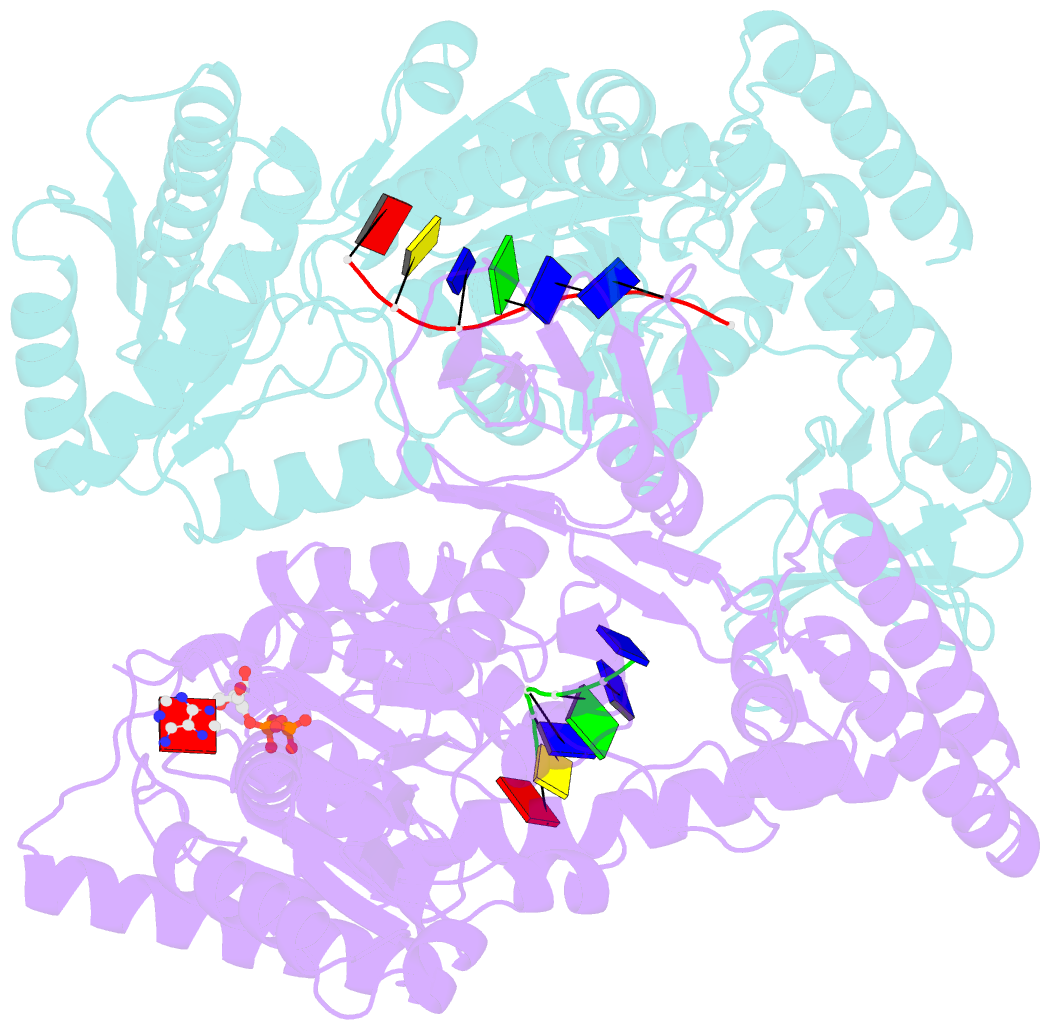
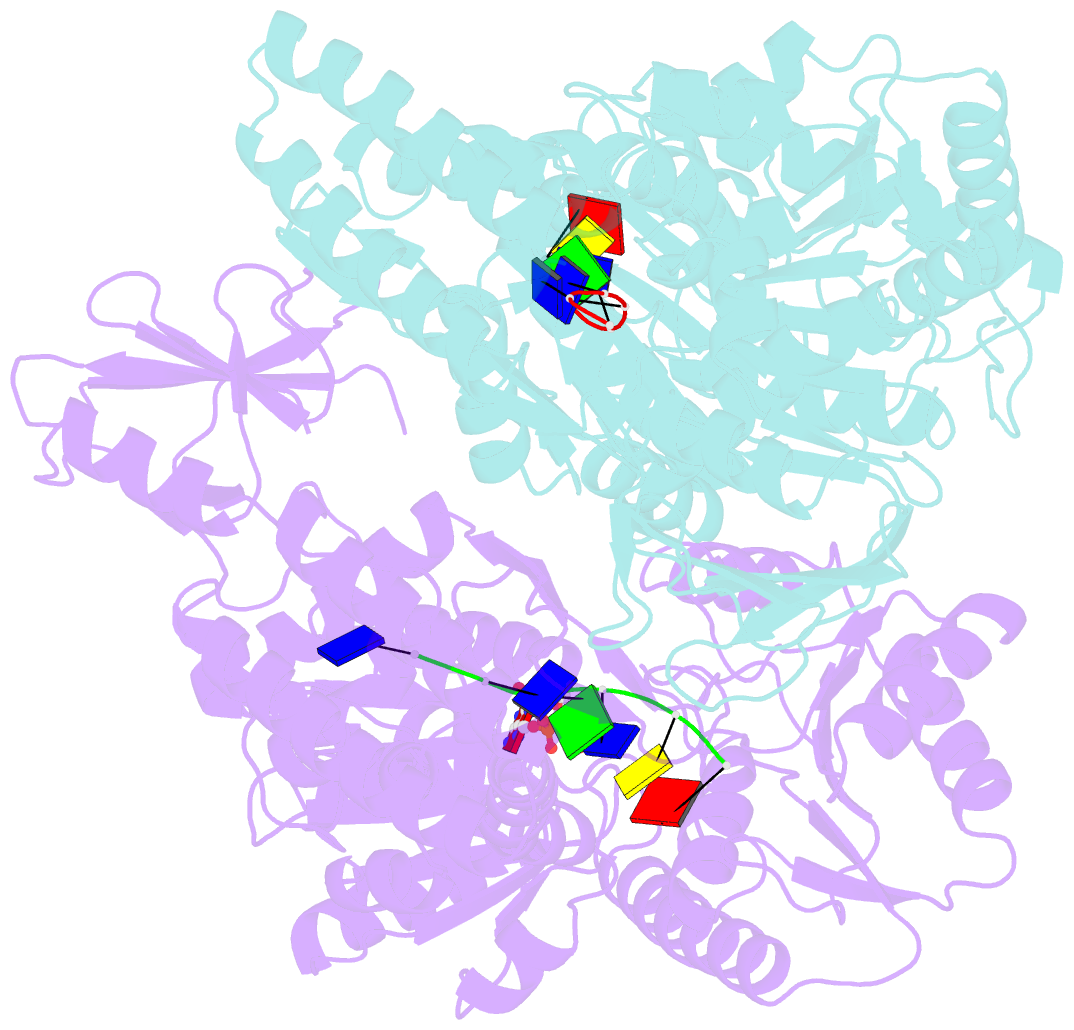
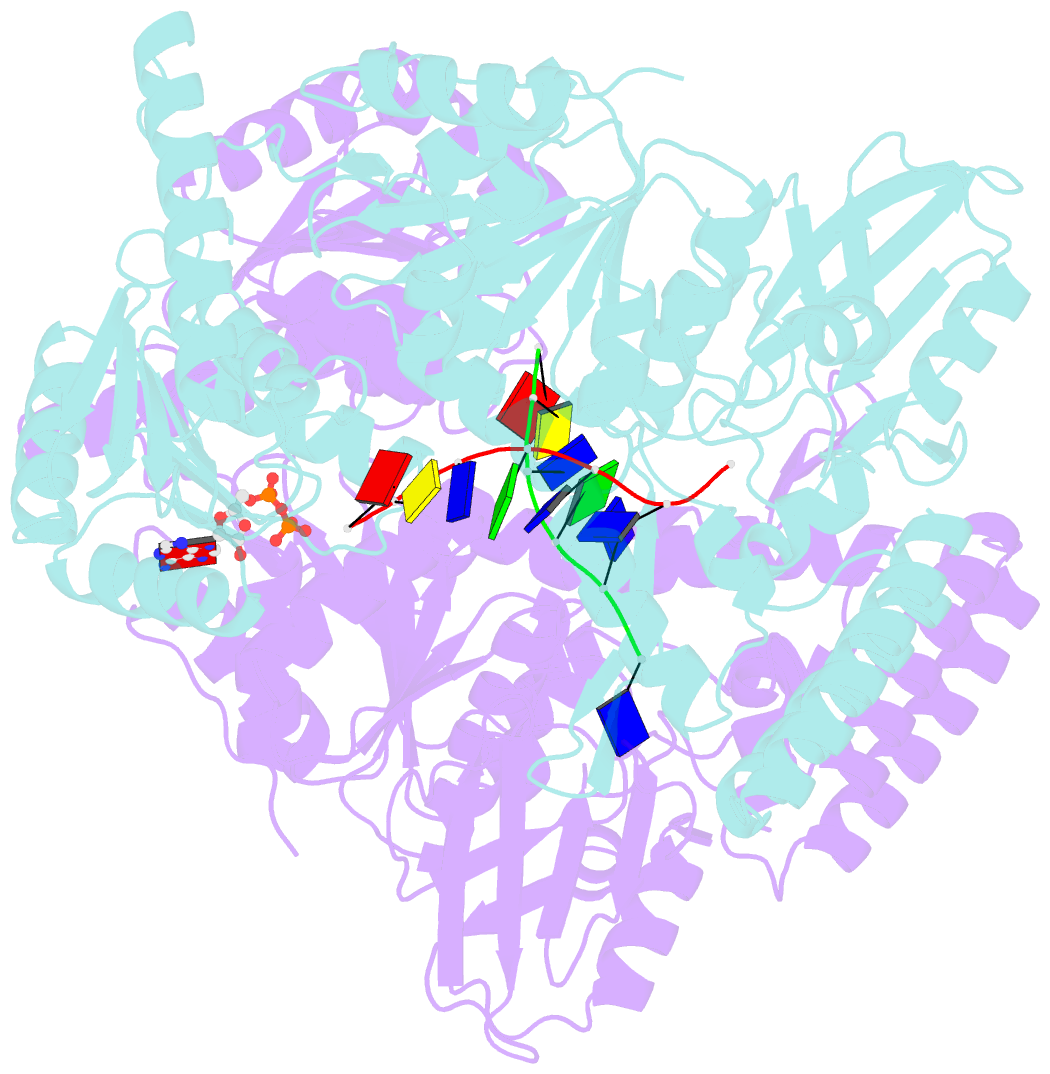
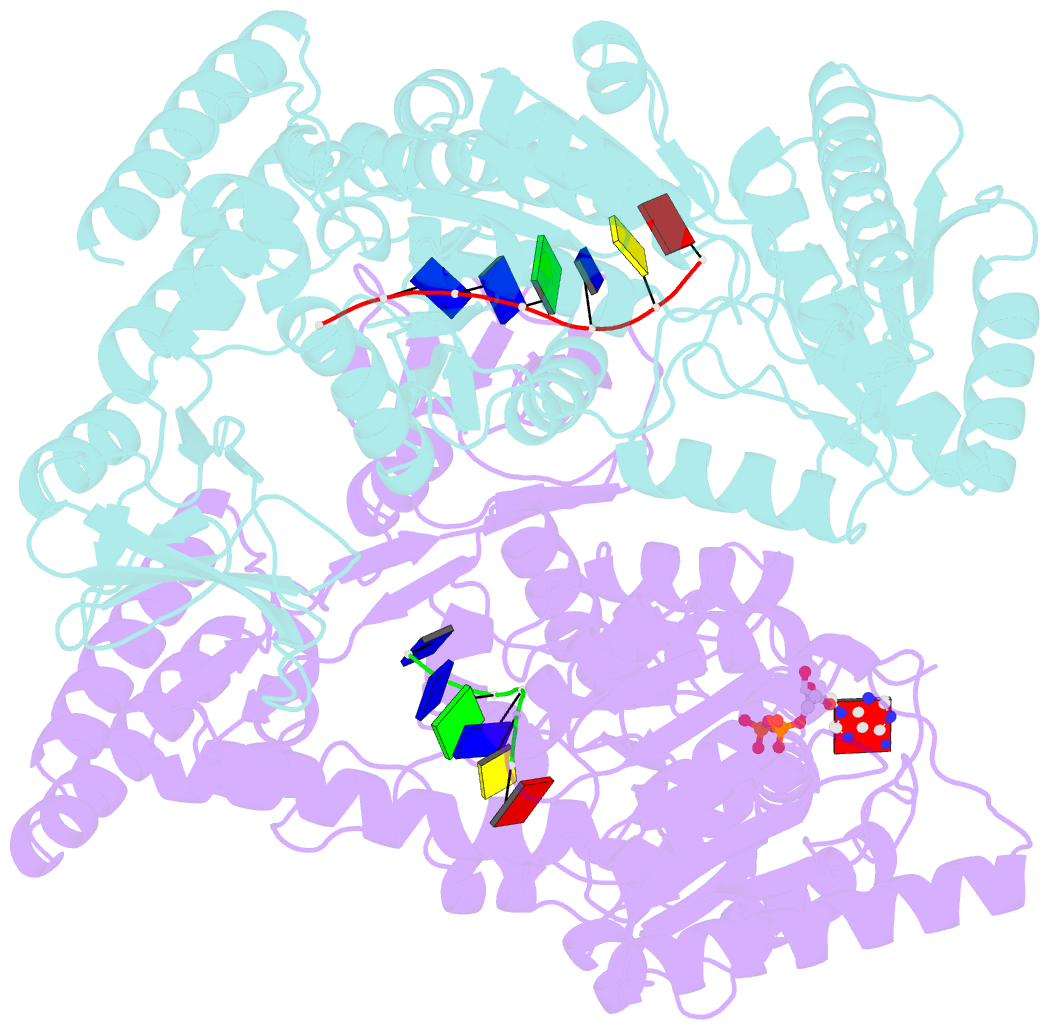
- Crystal structure of a uvrb dimer-DNA complex
- Reference
- Webster MP, Jukes R, Zamfir VS, Kay CW, Bagneris C, Barrett T (2012): "Crystal structure of the UvrB dimer: insights into the nature and functioning of the UvrAB damage engagement and UvrB-DNA complexes." Nucleic Acids Res., 40, 8743-8758. doi: 10.1093/nar/gks633.
- Abstract
- UvrB has a central role in the highly conserved UvrABC pathway functioning not only as a damage recognition element but also as an essential component of the lesion tracking machinery. While it has been recently confirmed that the tracking assembly comprises a UvrA2B2 heterotetramer, the configurations of the damage engagement and UvrB-DNA handover complexes remain obscure. Here, we present the first crystal structure of a UvrB dimer whose biological significance has been verified using both chemical cross-linking and electron paramagnetic resonance spectroscopy. We demonstrate that this dimeric species stably associates with UvrA and forms a UvrA2B2-DNA complex. Our studies also illustrate how signals are transduced between the ATP and DNA binding sites to generate the helicase activity pivotal to handover and formation of the UvrB2-DNA complex, providing key insights into the configurations of these important repair intermediates.