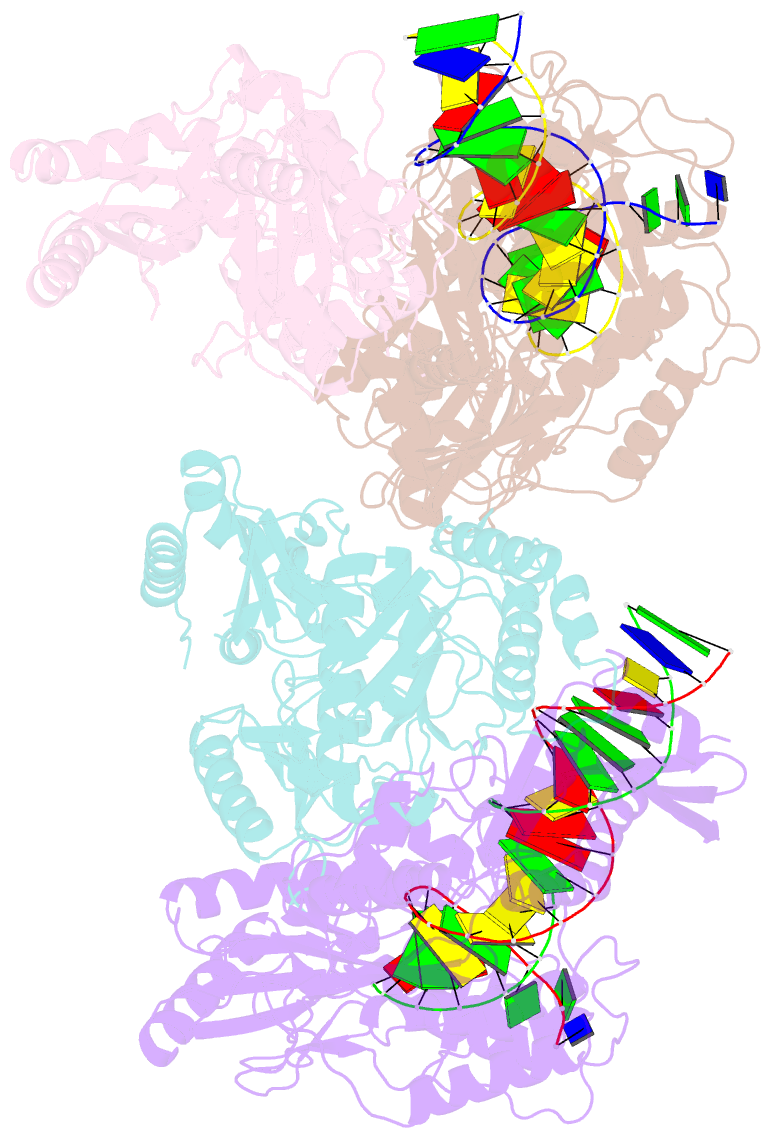
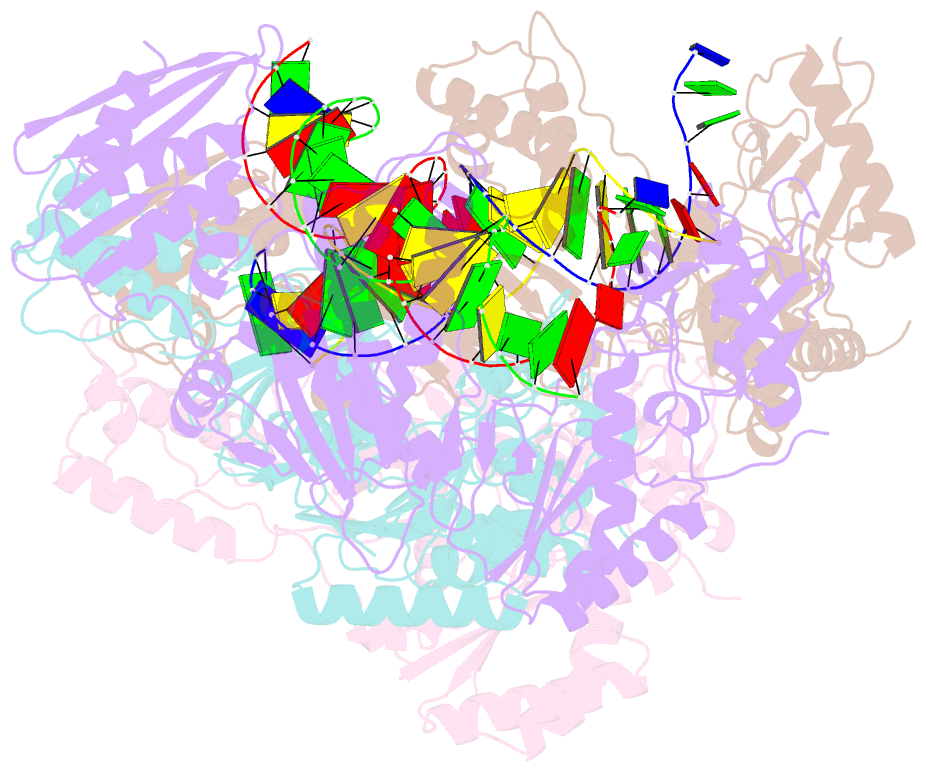
Summary information and primary citation
- PDB-id
- 3v6d; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.7 Å)
- Summary
- Crystal structure of hiv-1 reverse transcriptase (rt) cross-linked with azt-terminated DNA
- Reference
- Das K, Martinez SE, Bauman JD, Arnold E (2012): "HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism." Nat.Struct.Mol.Biol., 19, 253-259. doi: 10.1038/nsmb.2223.
- Abstract
- Combinations of nucleoside and non-nucleoside inhibitors (NNRTIs) of HIV-1 reverse transcriptase (RT) are widely used in anti-AIDS therapies. Five NNRTIs, including nevirapine, are clinical drugs; however, the molecular mechanism of inhibition by NNRTIs is not clear. We determined the crystal structures of RT-DNA-nevirapine, RT-DNA, and RT-DNA-AZT-triphosphate complexes at 2.85-, 2.70- and 2.80-Å resolution, respectively. The RT-DNA complex in the crystal could bind nevirapine or AZT-triphosphate but not both. Binding of nevirapine led to opening of the NNRTI-binding pocket. The pocket formation caused shifting of the 3' end of the DNA primer by ~5.5 Å away from its polymerase active site position. Nucleic acid interactions with fingers and palm subdomains were reduced, the dNTP-binding pocket was distorted and the thumb opened up. The structures elucidate complementary roles of nucleoside and non-nucleoside inhibitors in inhibiting RT.