Summary information and primary citation
- PDB-id
- 3vh0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- protein binding-DNA
- Method
- X-ray (2.9 Å)
- Summary
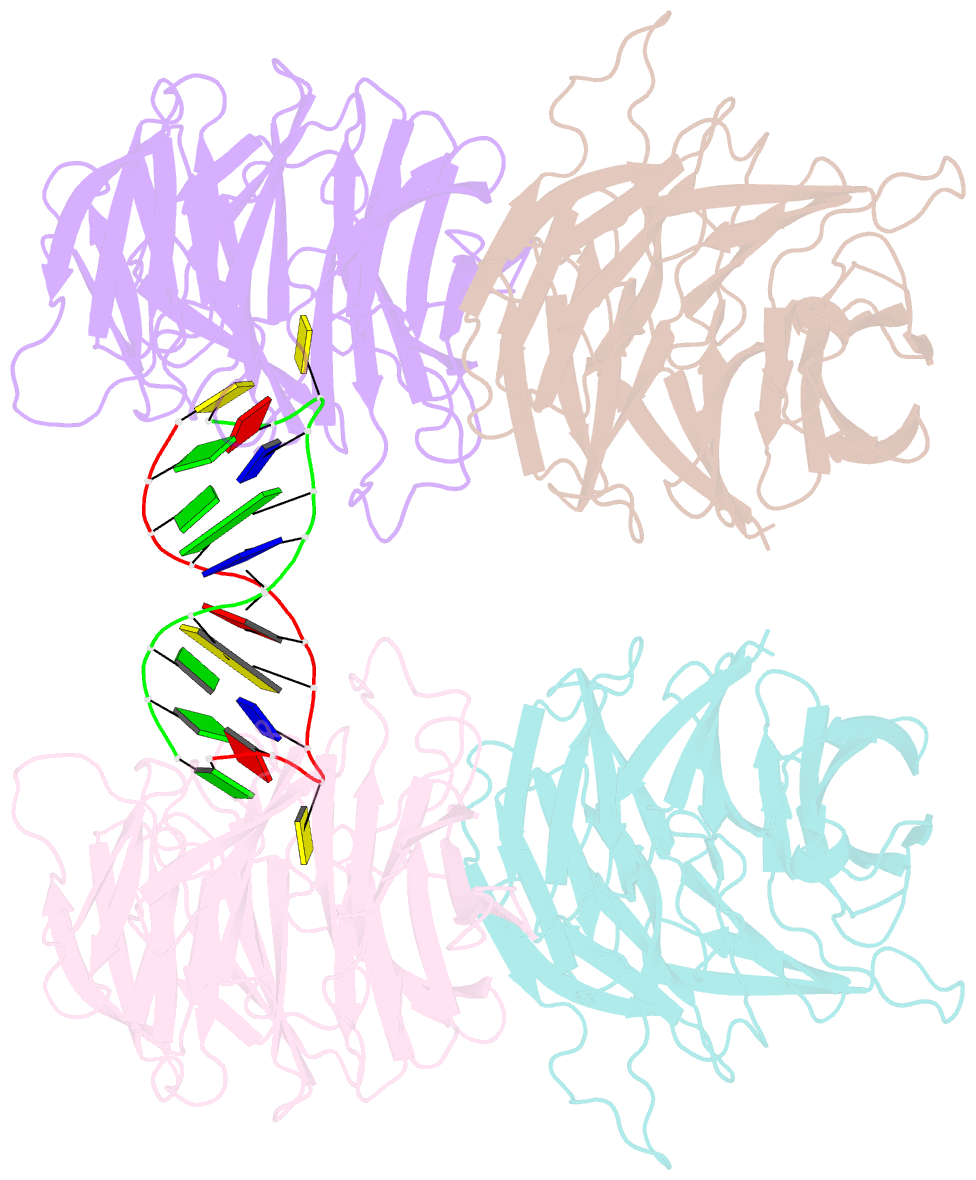
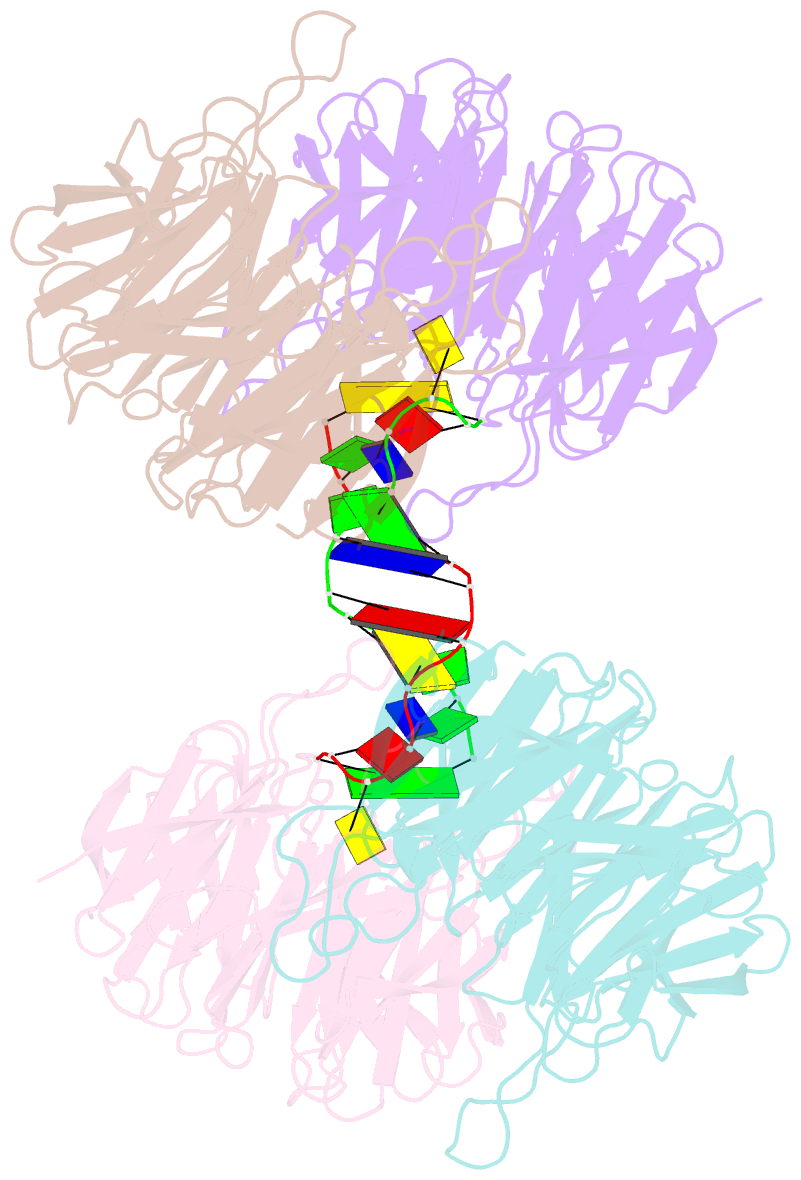
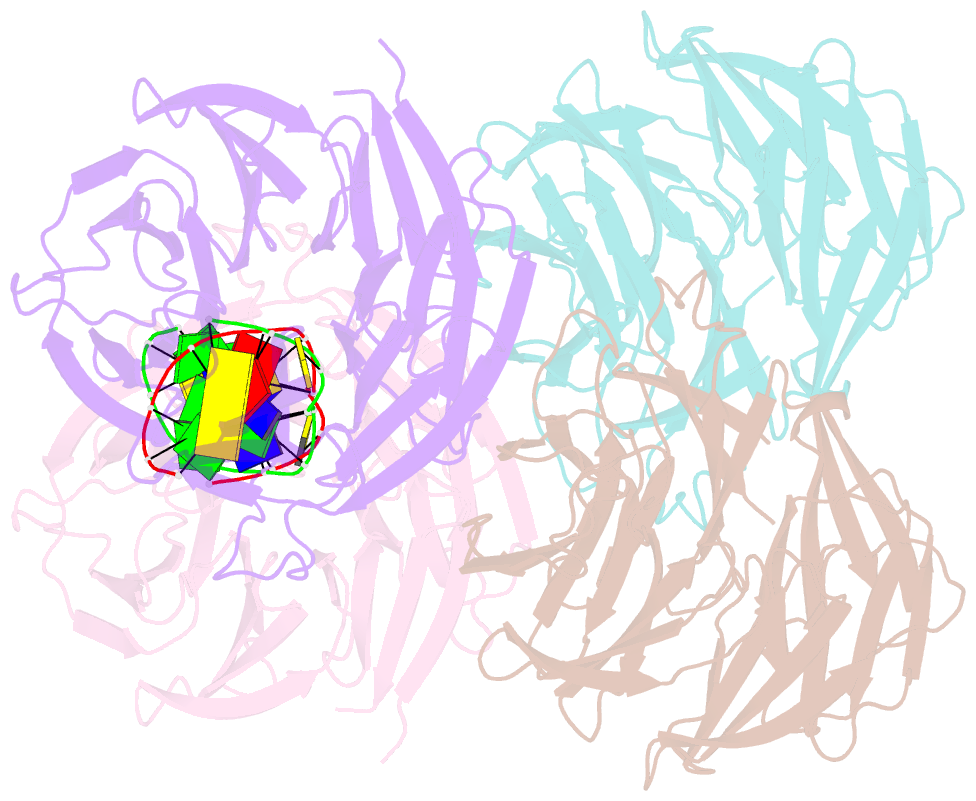
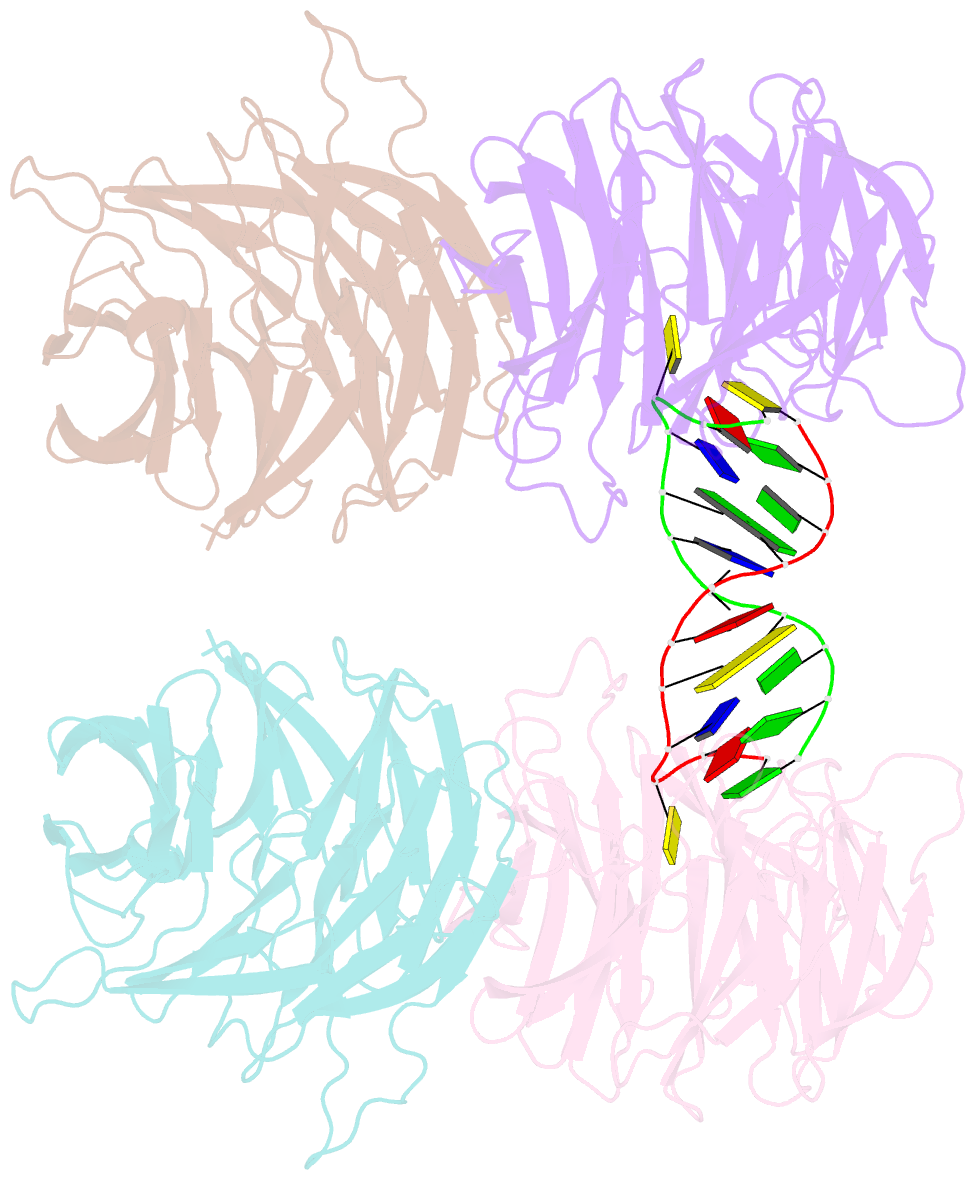
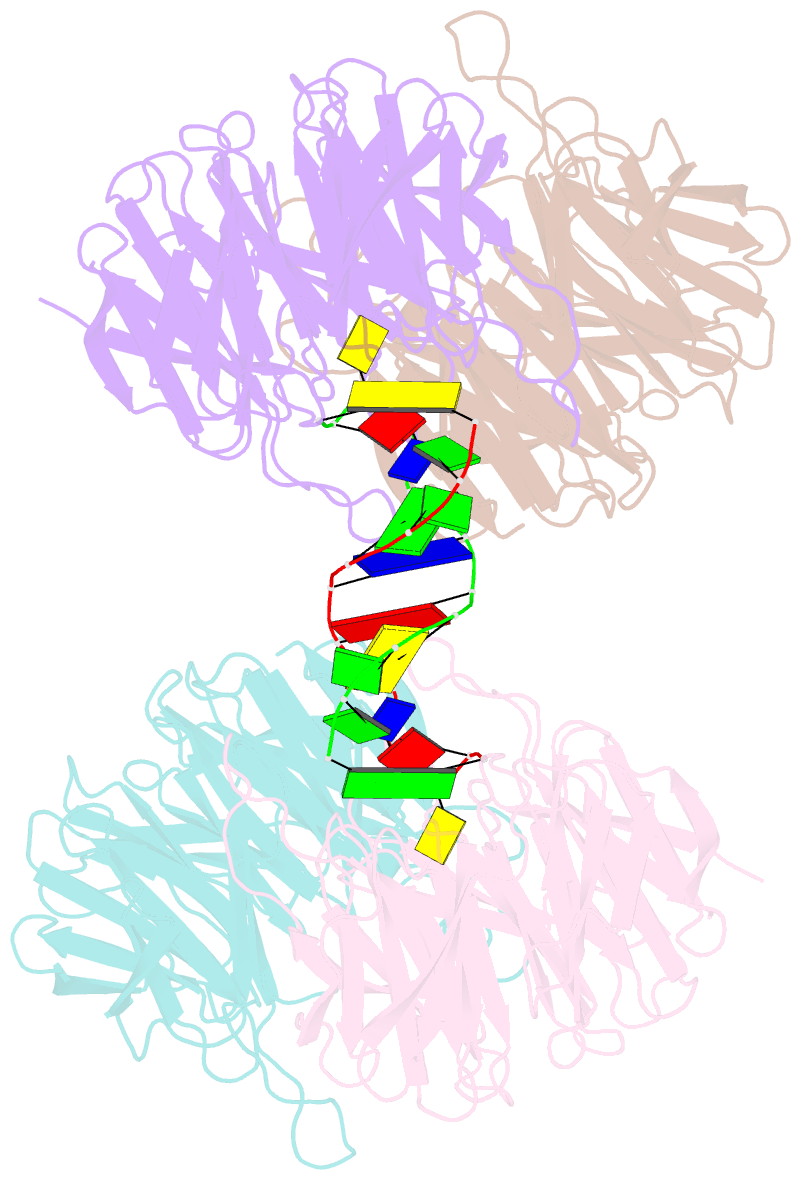
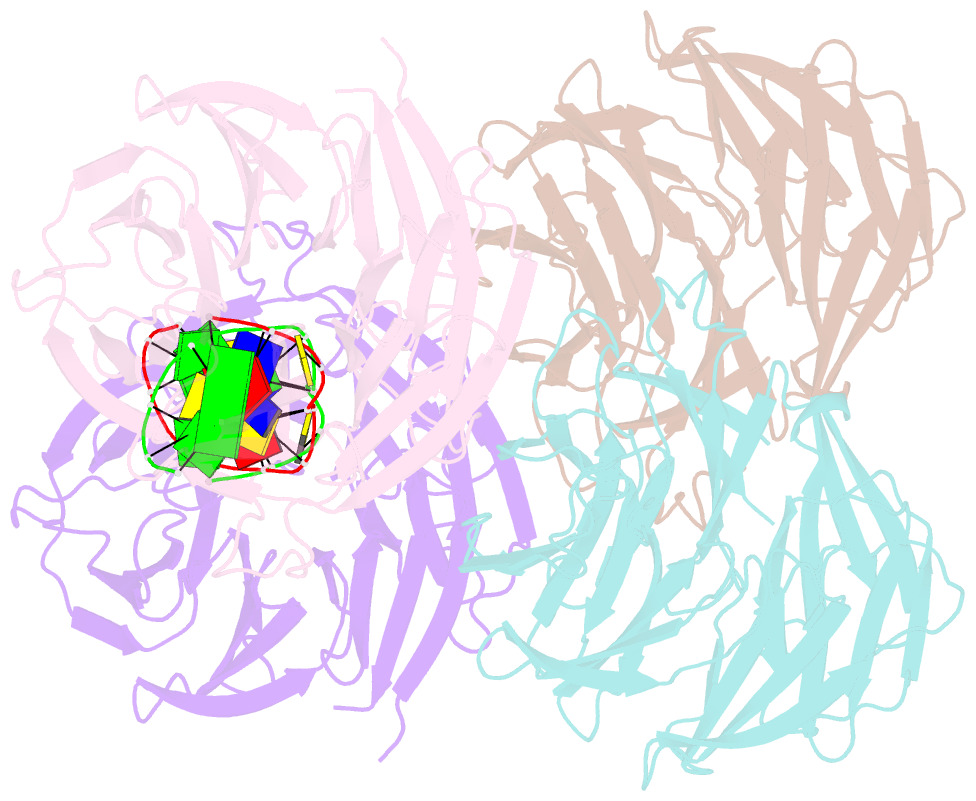
- Crystal structure of e. coli ynce complexed with DNA
- Reference
- Kagawa W, Sagawa T, Niki H, Kurumizaka H (2011): "Structural basis for the DNA-binding activity of the bacterial beta-propeller protein YncE." Acta Crystallogr.,Sect.D, 67, 1045-1053. doi: 10.1107/S0907444911045033.
- Abstract
- β-Propellers are widely utilized in nature as recognition modules. The well conserved β-propeller fold exhibits a high degree of functional diversity, which is probably accomplished through variations in the surface properties of the proteins. Little is known about the interactions between β-propeller proteins and nucleic acids. In the present study, it has been found that the bacterial β-propeller protein YncE binds to DNA. Crystal structures of YncE in the free form and complexed with DNA revealed that the surface region of YncE corresponding to the `canonical' substrate-binding site forms essential contacts with DNA. A single DNA base within a single-stranded DNA region is trapped in the hydrophobic pocket located within the central channel of the β-propeller protein. These data provide physical evidence for the DNA-binding ability of the previously uncharacterized YncE and also suggest that the `canonical' substrate-binding site may be commonly adapted to facilitate nucleic acid binding in a subset of β-propeller proteins.