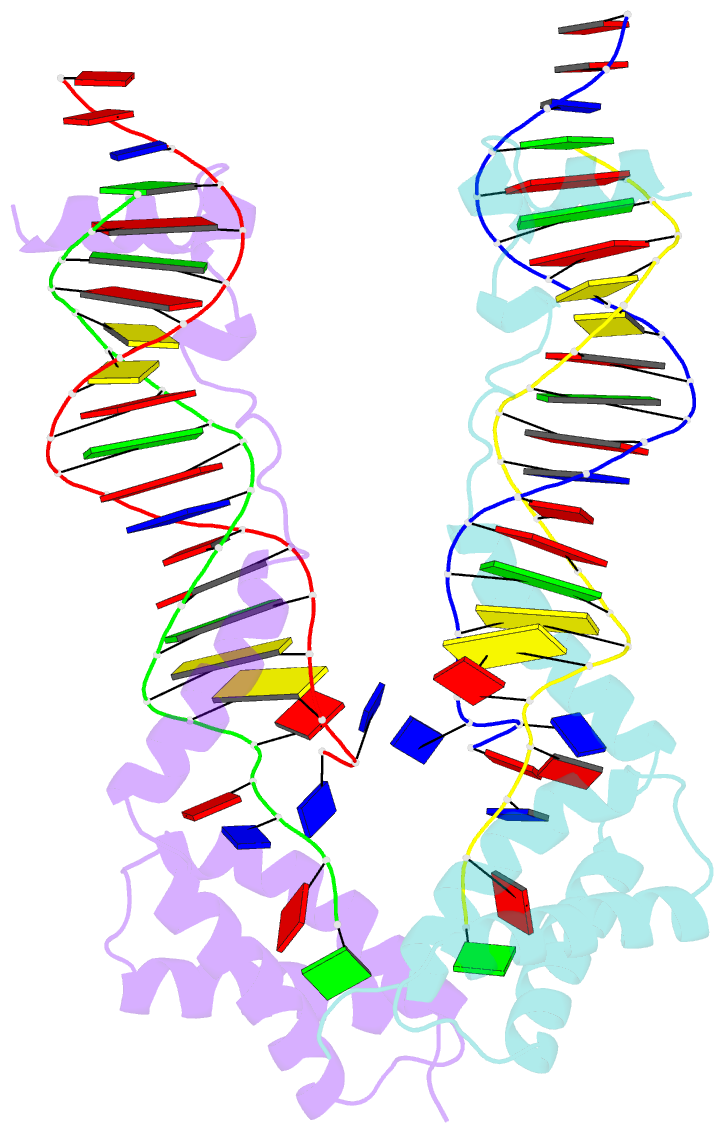
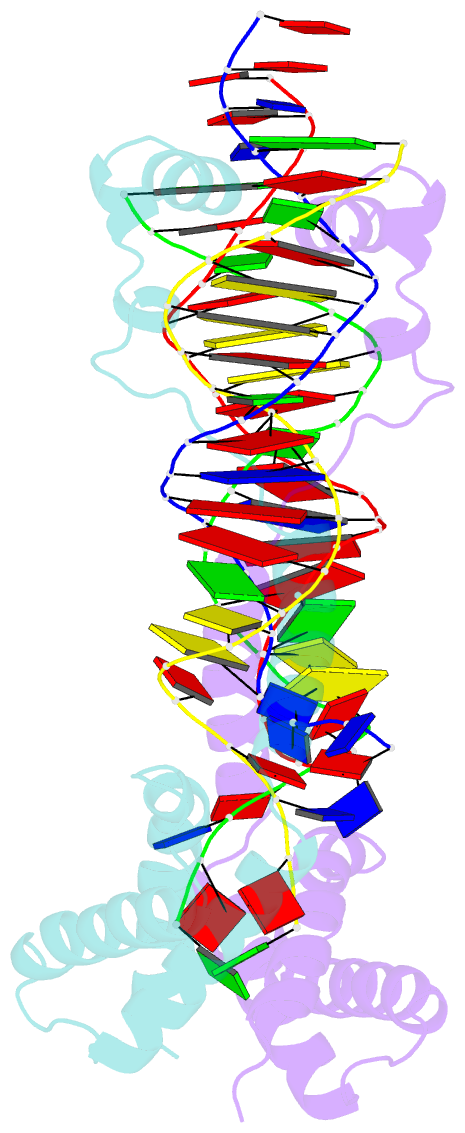
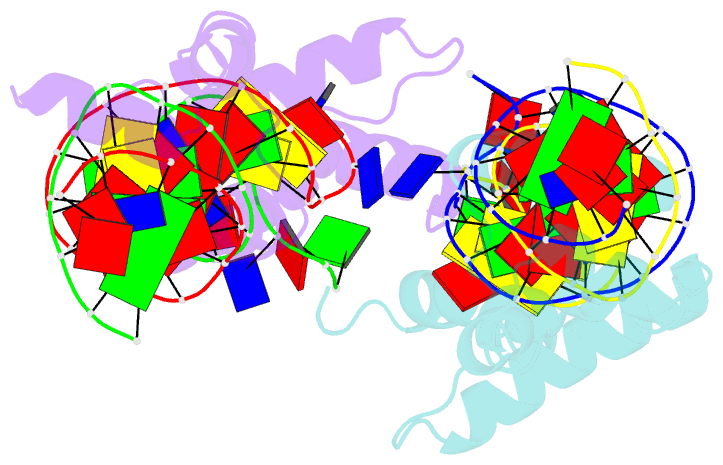
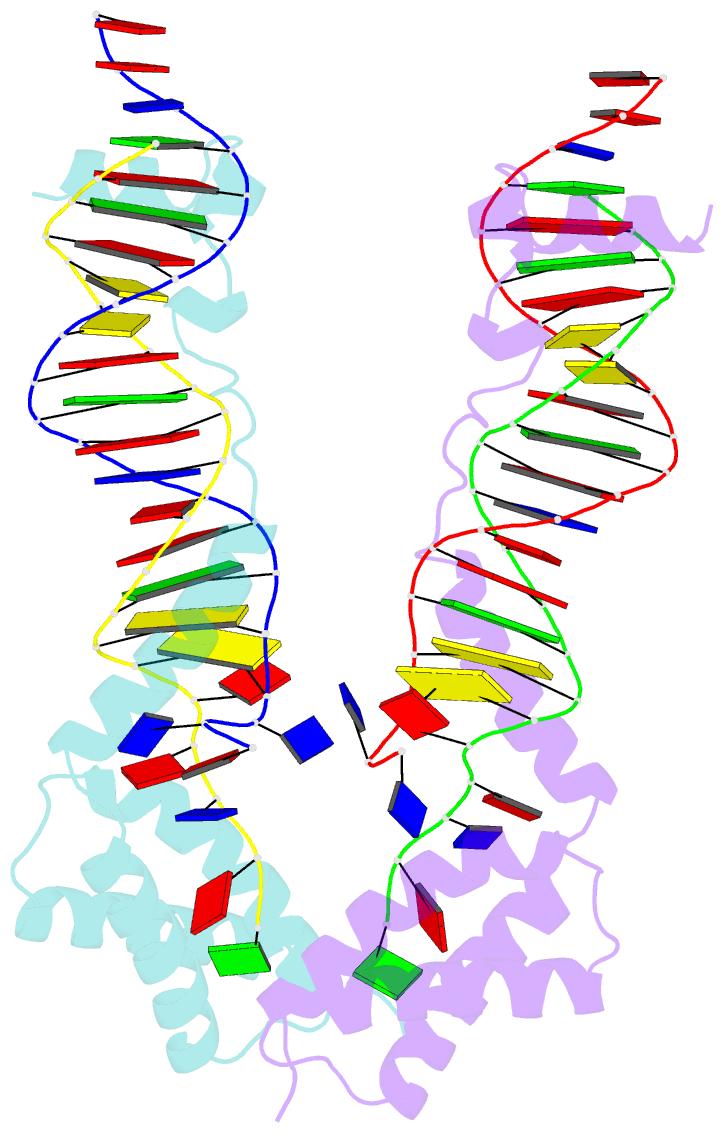
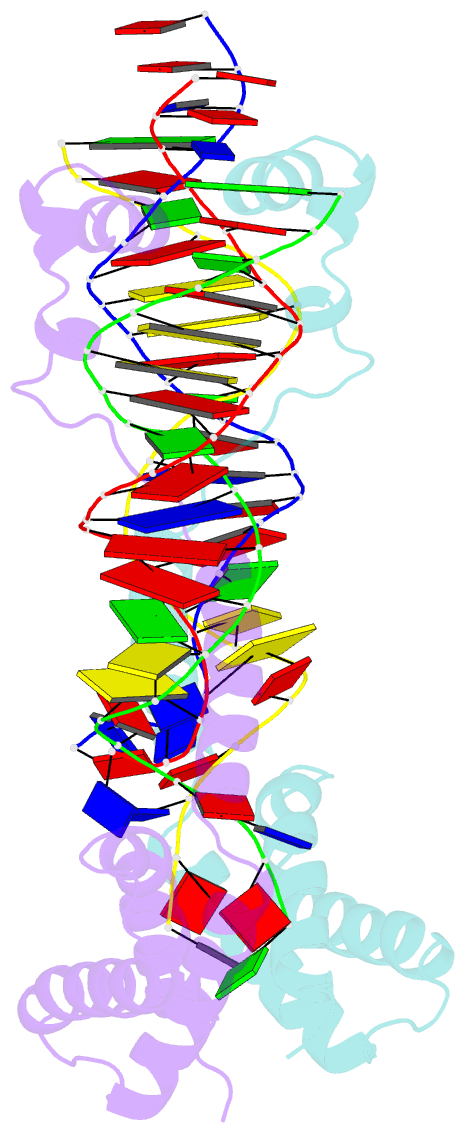
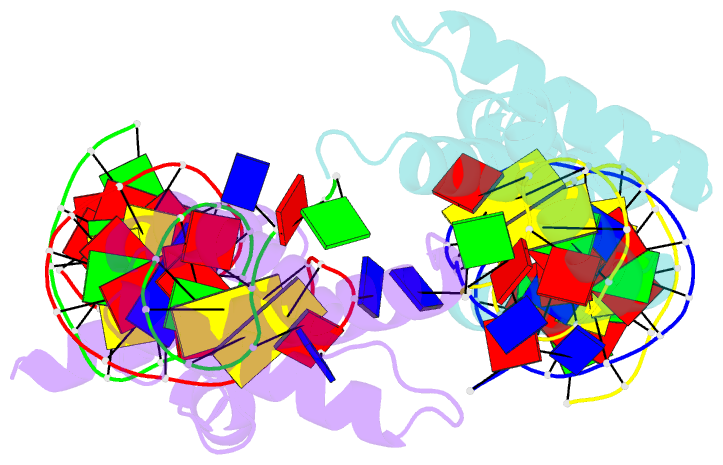
Summary information and primary citation
- PDB-id
-
3vw4;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.7 Å)
- Summary
- Crystal structure of the DNA-binding domain of cole2-p9
rep in complex with the replication origin
- Reference
-
Itou H, Yagura M, Shirakihara Y, Itoh T (2015): "Structural
Basis for Replication Origin Unwinding by An
Initiator-Primase of Plasmid ColE2-P9: Duplex DNA
Unwinding by A Single Protein."
J.Biol.Chem., 290, 3601-3611.
doi: 10.1074/jbc.M114.595645.
- Abstract
- Duplex DNA is generally unwound by protein oligomers
prior to replication. The Rep protein of plasmid ColE2-P9
(34 kDa) is an essential initiator for plasmid DNA
replication. This protein binds the replication origin
(Ori) in a sequence-specific manner as a monomer and
unwinds DNA. Here we present the crystal structure of the
DNA-binding domain of Rep (E2Rep-DBD) in complex with Ori
DNA. The structure unveils the basis for Ori-specific
recognition by the E2Rep-DBD and also reveals that it
unwinds DNA by the concerted actions of its three
contiguous structural modules. The structure also shows
that the functionally unknown PriCT domain, which forms a
compact module, plays a central role in DNA unwinding. The
conservation of the PriCT domain in the C termini of some
archaeo-eukaryotic primases indicates that it probably
plays a similar role in these proteins. Thus, this is the
first report providing the structural basis for the
functional importance of the conserved PriCT domain and
also reveals a novel mechanism for DNA unwinding by a
single protein.