Summary information and primary citation
- PDB-id
- 3waz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.0 Å)
- Summary
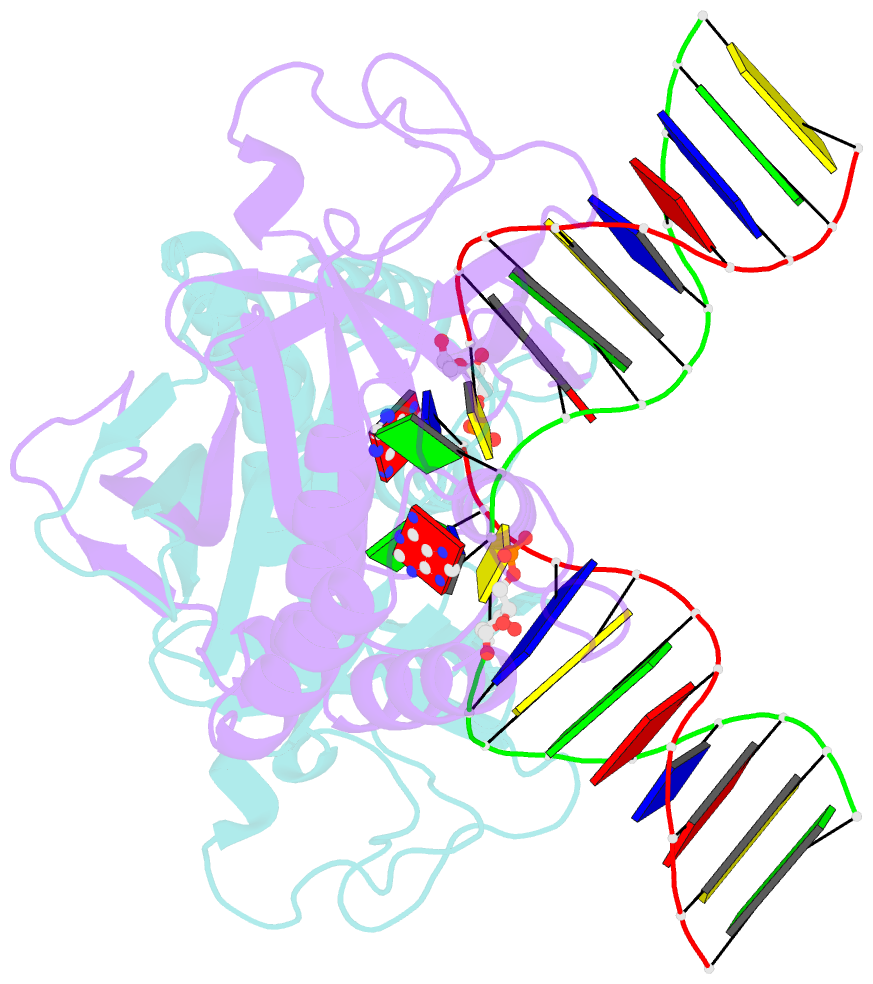
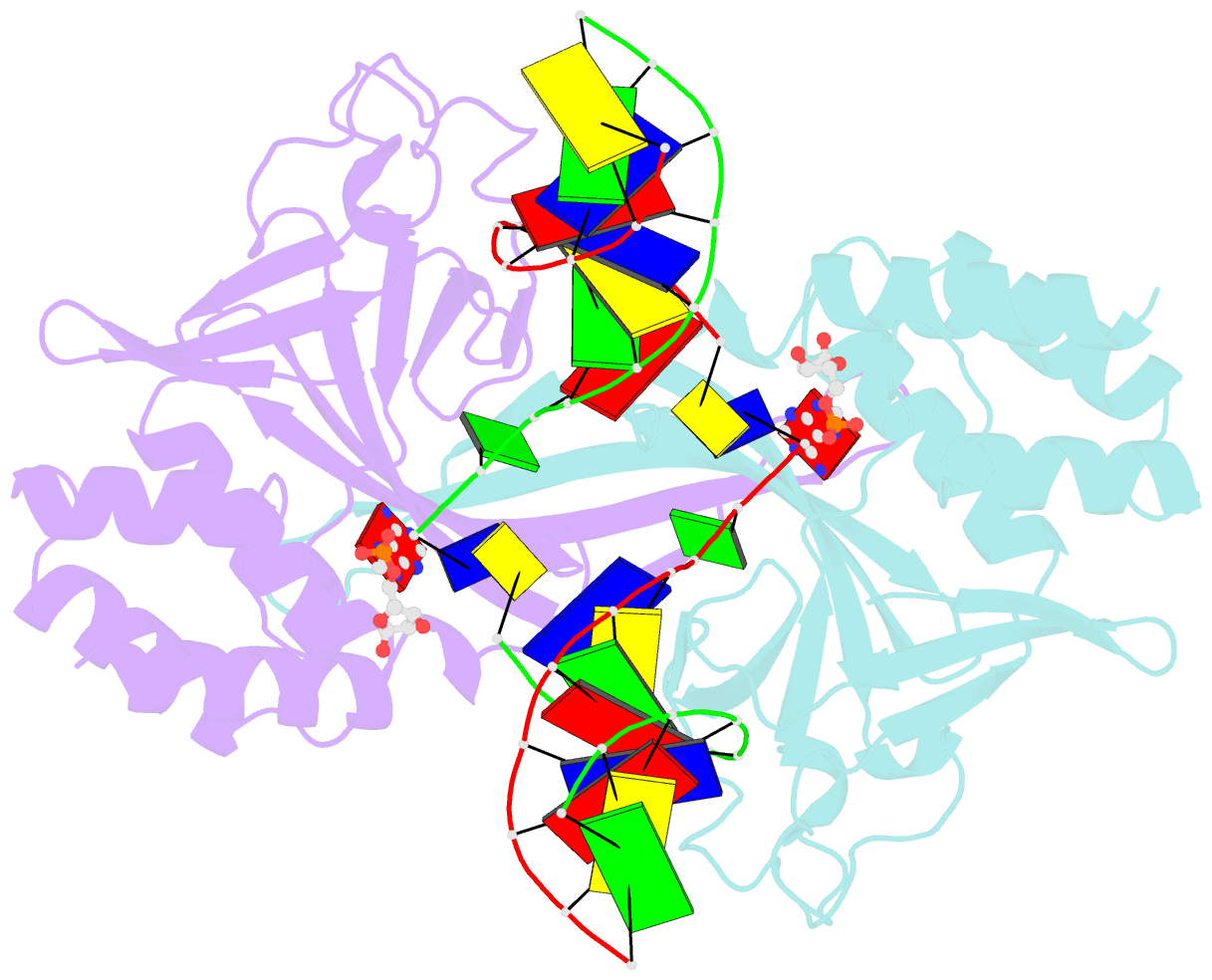
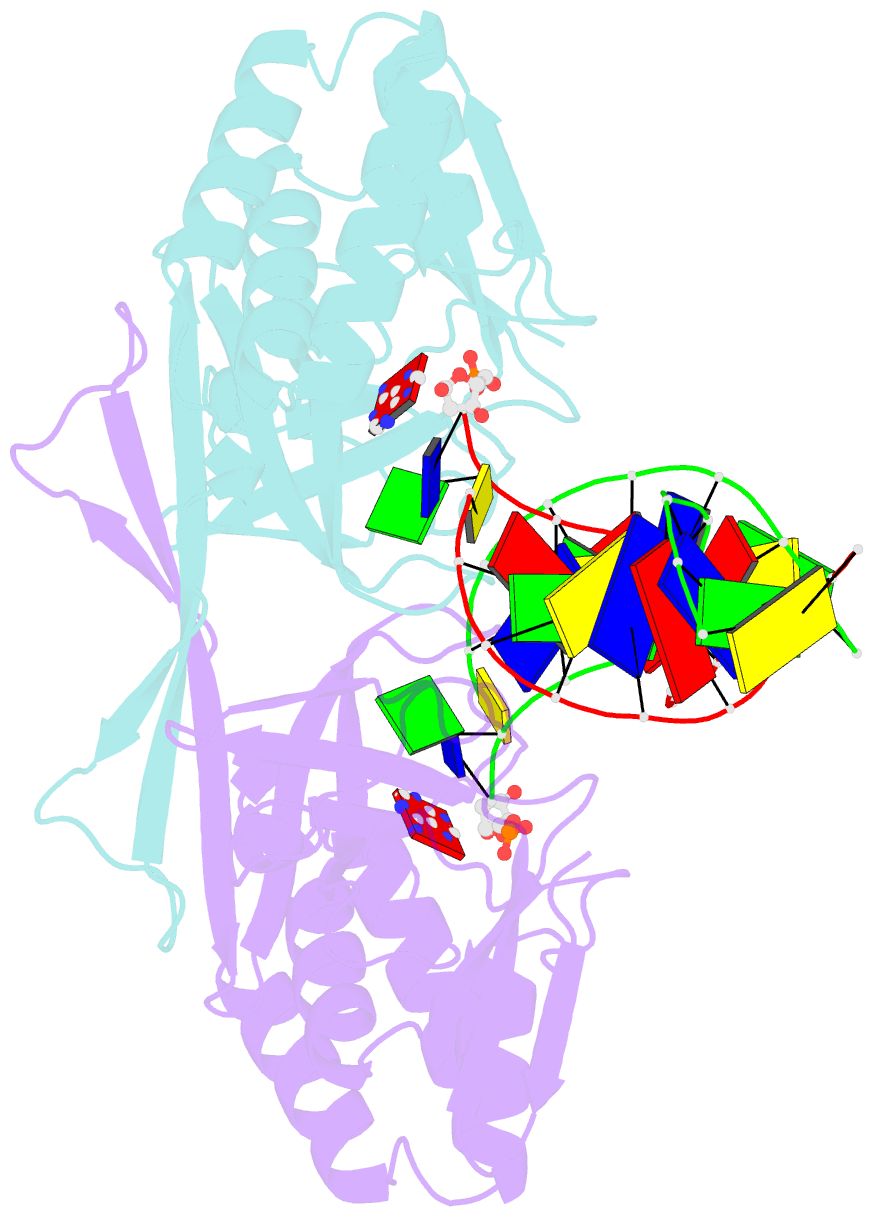
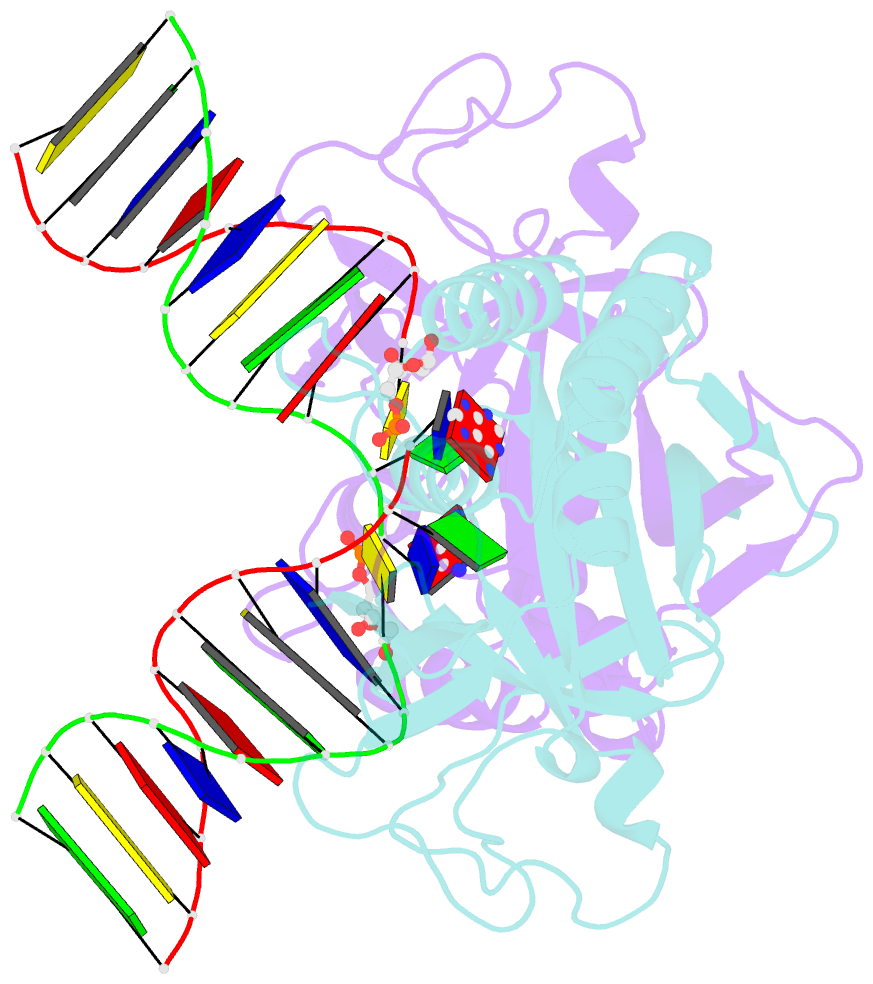
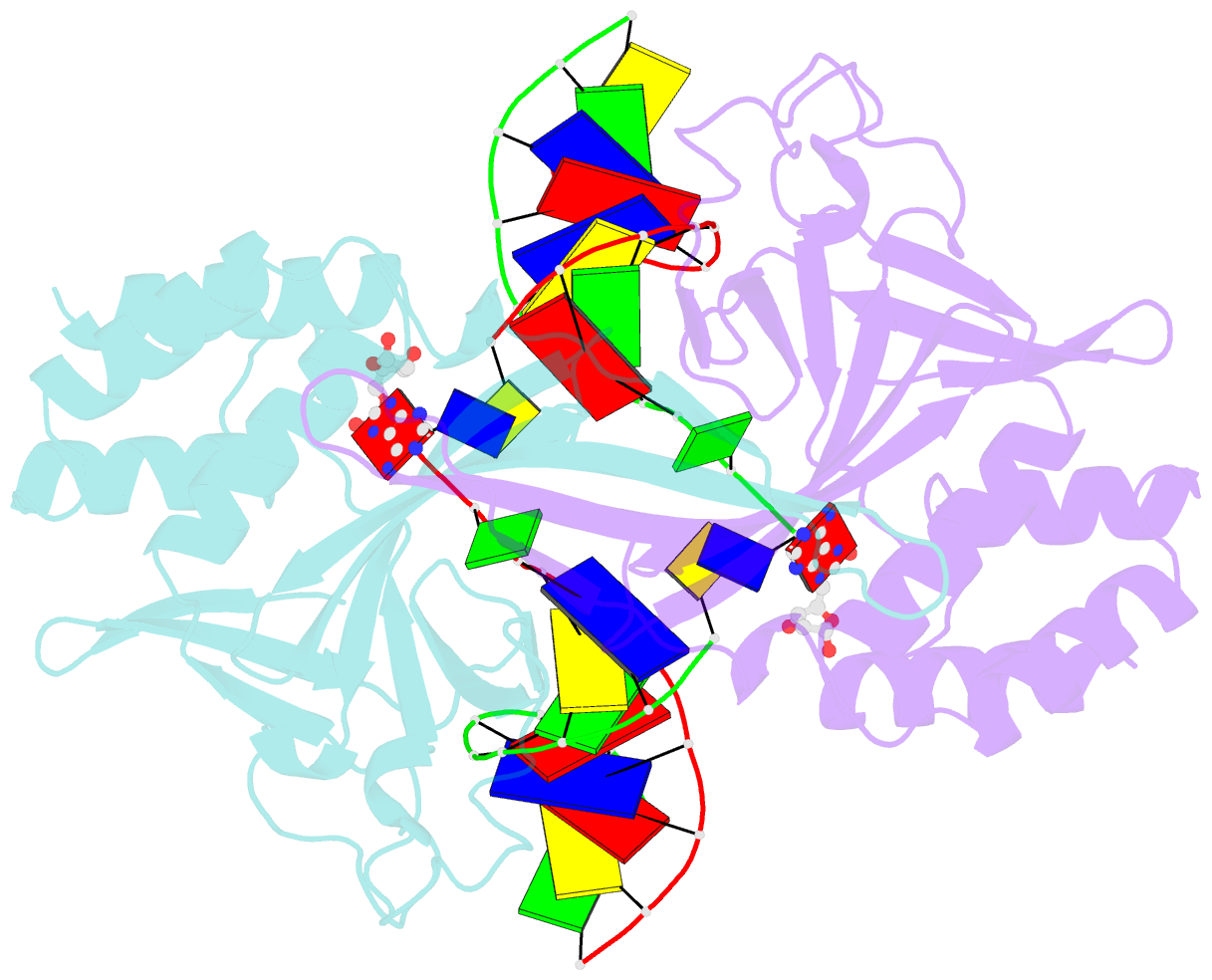
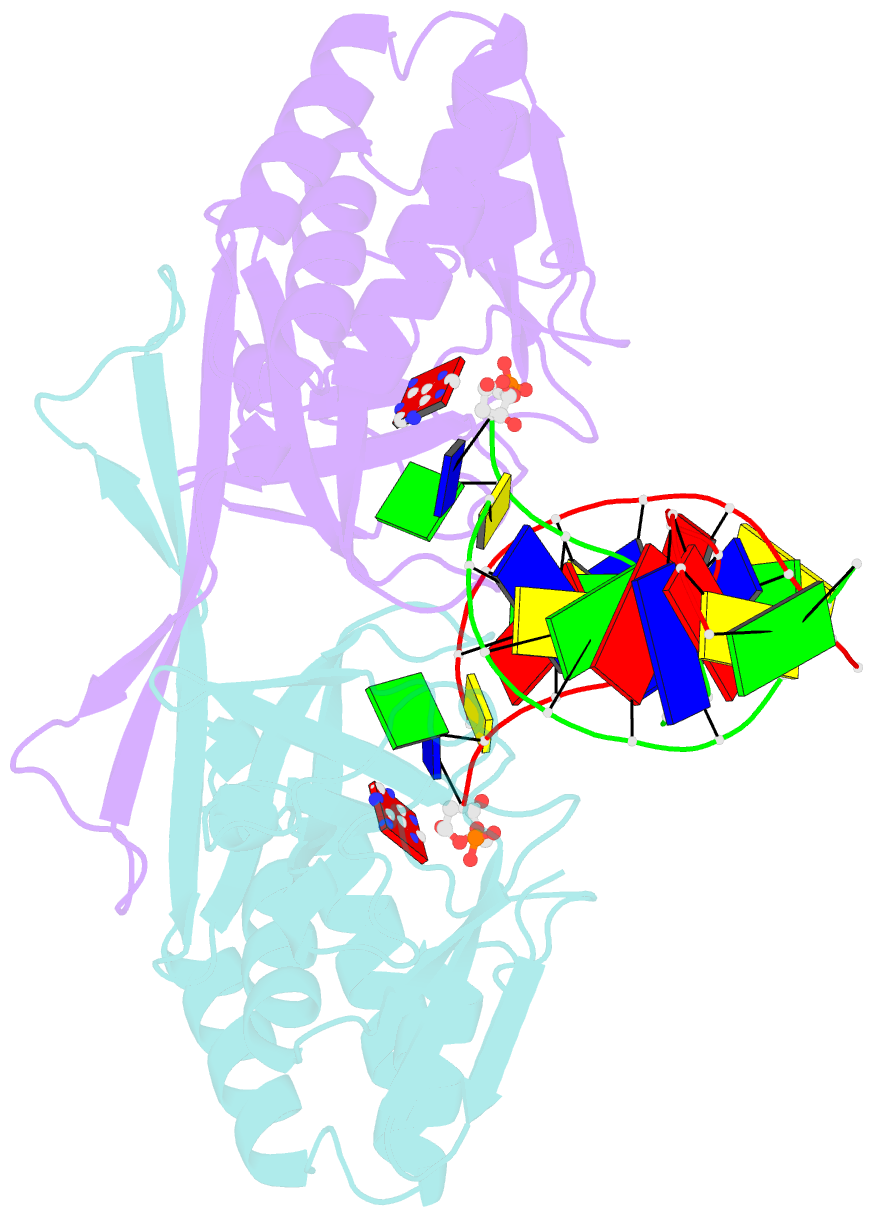
- Crystal structure of a restriction enzyme pabi in complex with DNA
- Reference
- Miyazono K, Furuta Y, Watanabe-Matsui M, Miyakawa T, Ito T, Kobayashi I, Tanokura M (2014): "A sequence-specific DNA glycosylase mediates restriction-modification in Pyrococcus abyssi." Nat Commun, 5, 3178. doi: 10.1038/ncomms4178.
- Abstract
- Restriction-modification systems consist of genes that encode a restriction enzyme and a cognate methyltransferase. Thus far, it was believed that restriction enzymes are sequence-specific endonucleases that introduce double-strand breaks at specific sites by catalysing the cleavages of phosphodiester bonds. Here we report that based on the crystal structure and enzymatic activity, one of the restriction enzymes, R.PabI, is not an endonuclease but a sequence-specific adenine DNA glycosylase. The structure of the R.PabI-DNA complex shows that R.PabI unwinds DNA at a 5'-GTAC-3' site and flips the guanine and adenine bases out of the DNA helix to recognize the sequence. R.PabI catalyses the hydrolysis of the N-glycosidic bond between the adenine base and the sugar in the DNA and produces two opposing apurinic/apyrimidinic (AP) sites. The opposing AP sites are cleaved by heat-promoted β elimination and/or by endogenous AP endonucleases of host cells to introduce a double-strand break.