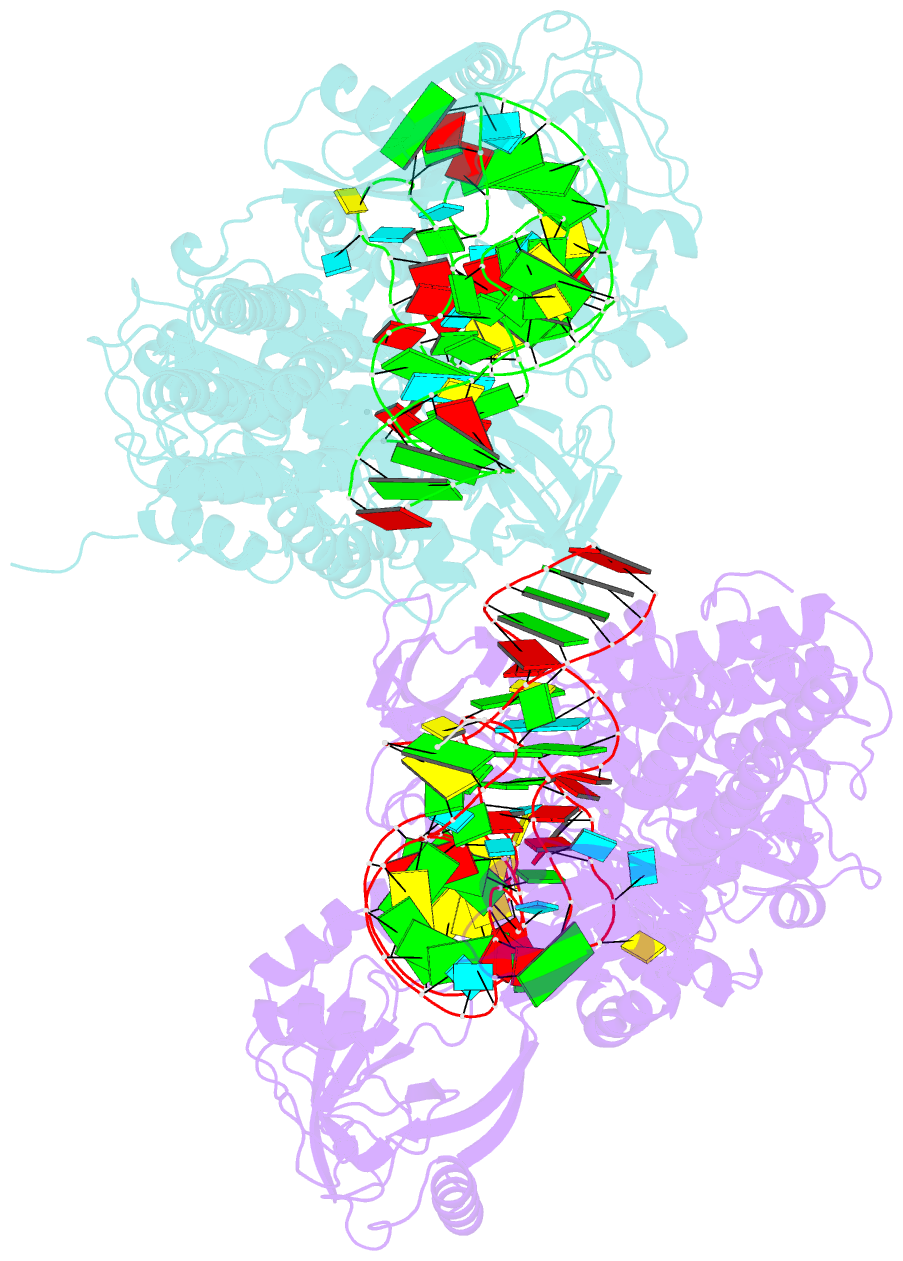
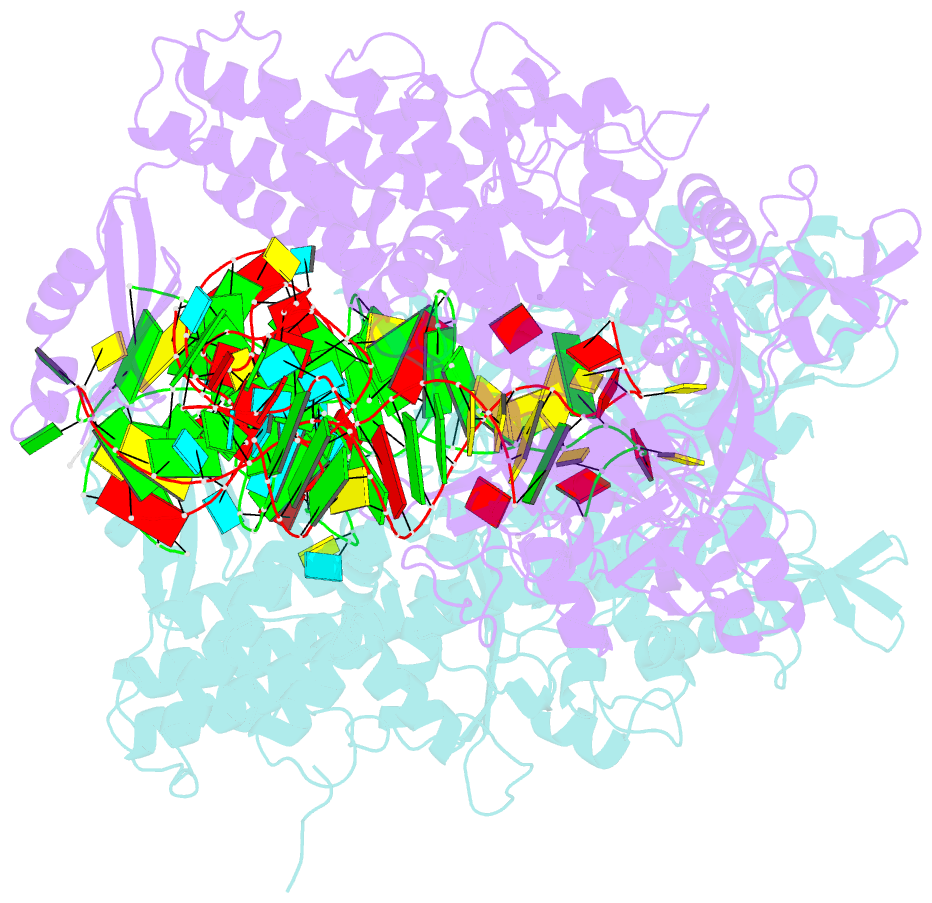
Summary information and primary citation
- PDB-id
- 3zgz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- X-ray (2.4 Å)
- Summary
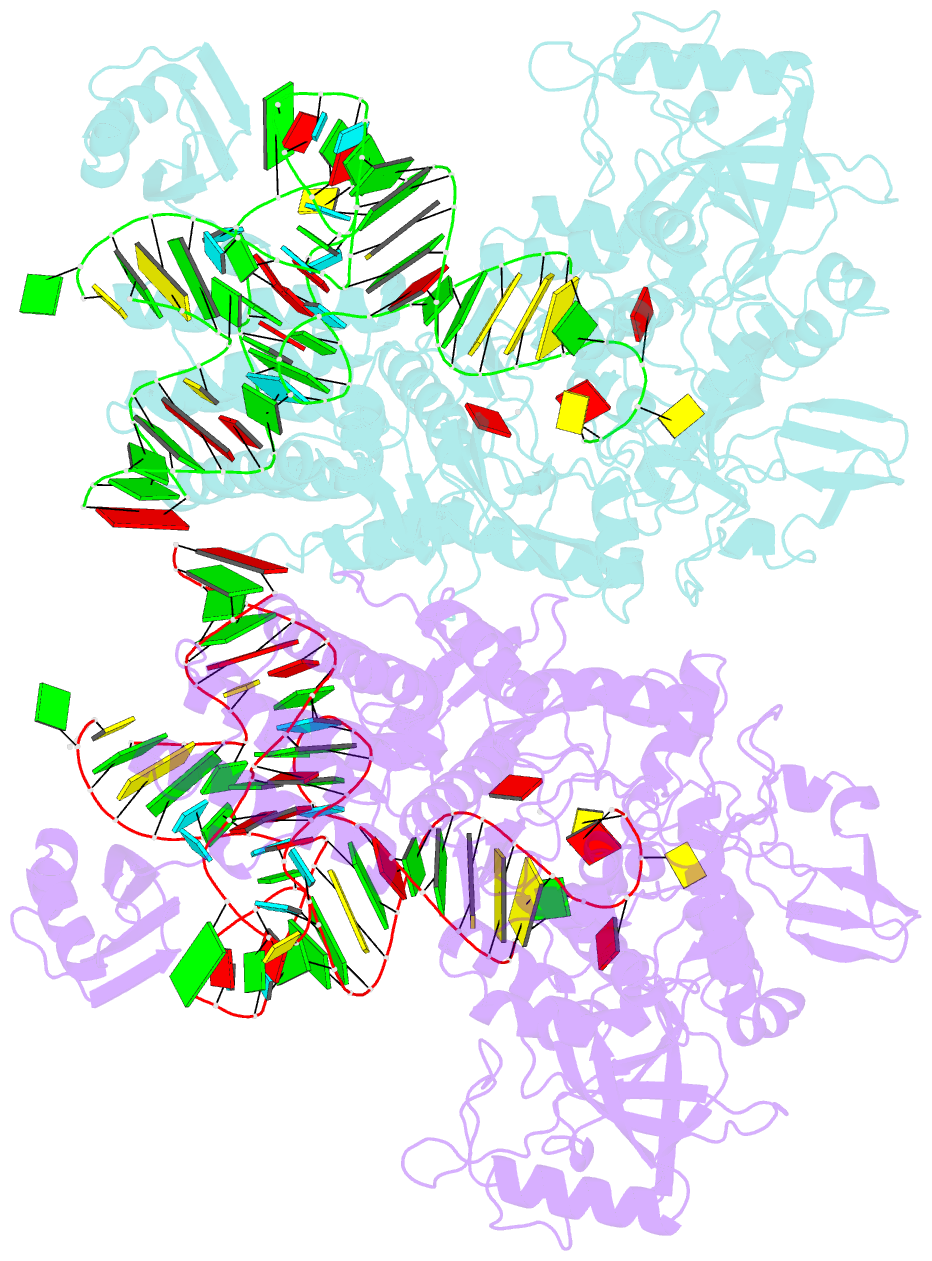
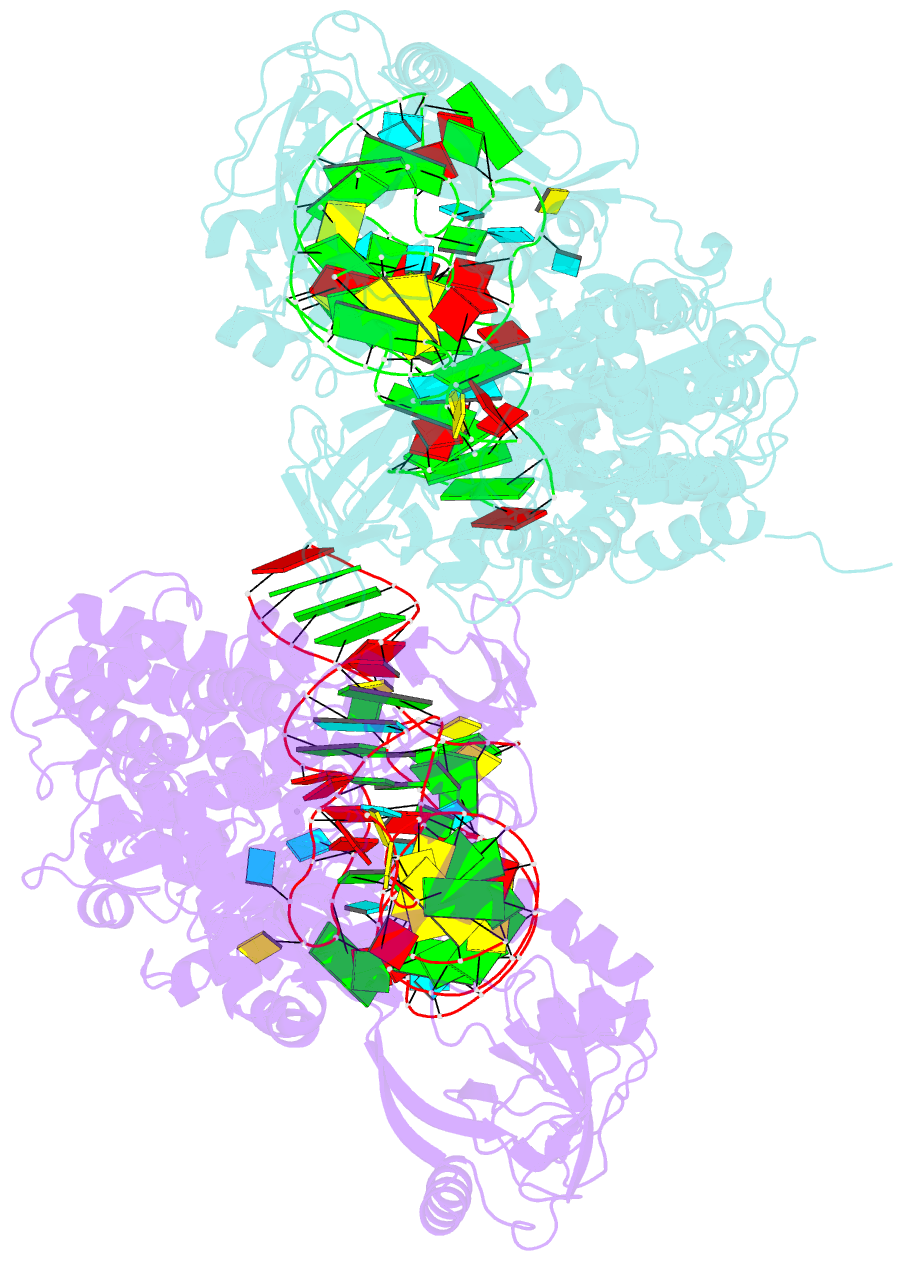
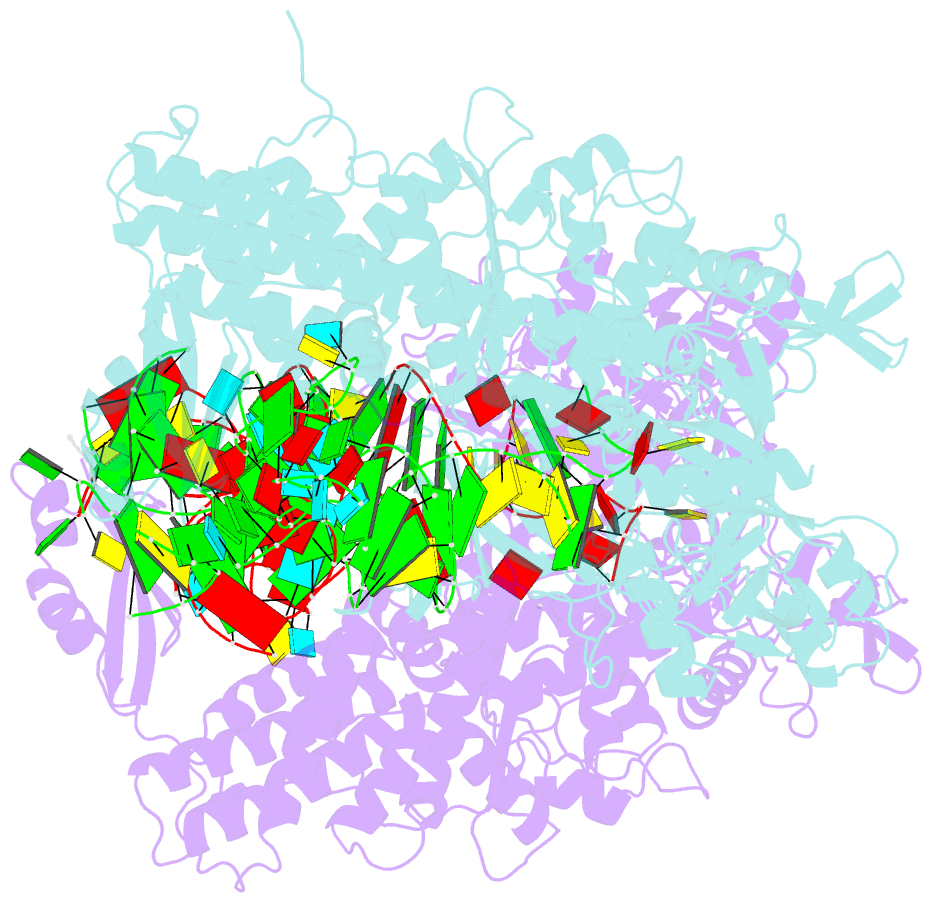
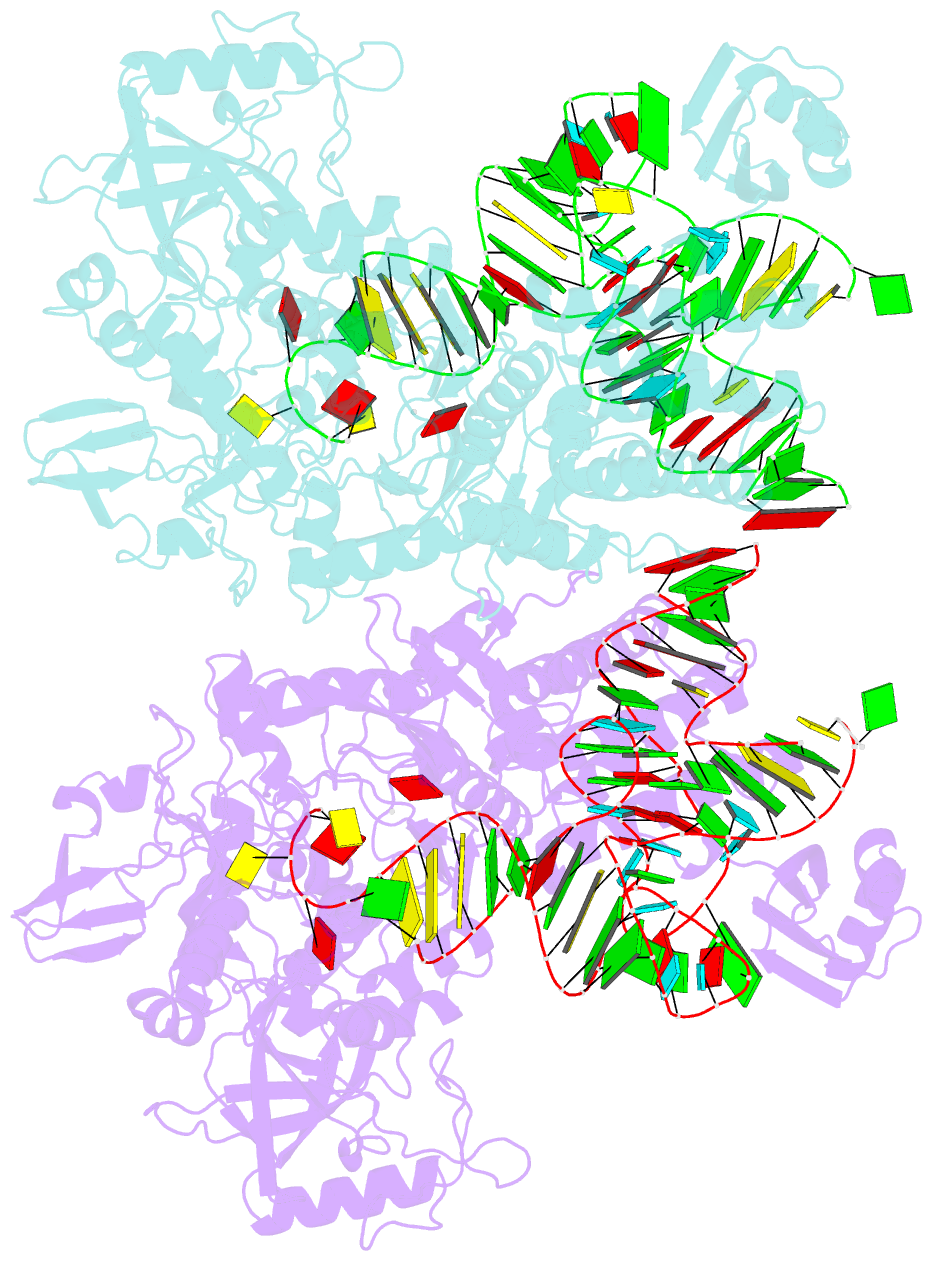
- Ternary complex of e. coli leucyl-trna synthetase, trna(leu) and toxic moiety from agrocin 84 (tm84) in aminoacylation-like conformation
- Reference
- Chopra S, Palencia A, Virus C, Tripathy A, Temple BR, Velazquez-Campoy A, Cusack S, Reader JS (2013): "Plant Tumour Biocontrol Agent Employs a tRNA-Dependent Mechanism to Inhibit Leucyl-tRNA Synthetase." Nat.Commun., 4, 1417. doi: 10.1038/NCOMMS2421.
- Abstract
- Leucyl-tRNA synthetases (LeuRSs) have an essential role in translation and are promising targets for antibiotic development. Agrocin 84 is a LeuRS inhibitor produced by the biocontrol agent Agrobacterium radiobacter K84 that targets pathogenic strains of A. tumefaciens, the causative agent of plant tumours. Agrocin 84 acts as a molecular Trojan horse and is processed inside the pathogen into a toxic moiety (TM84). Here we show using crystal structure, thermodynamic and kinetic analyses, that this natural antibiotic employs a unique and previously undescribed mechanism to inhibit LeuRS. TM84 requires tRNA(Leu) for tight binding to the LeuRS synthetic active site, unlike any previously reported inhibitors. TM84 traps the enzyme-tRNA complex in a novel 'aminoacylation-like' conformation, forming novel interactions with the KMSKS loop and the tRNA 3'-end. Our findings reveal an intriguing tRNA-dependent inhibition mechanism that may confer a distinct evolutionary advantage in vivo and inform future rational antibiotic design.