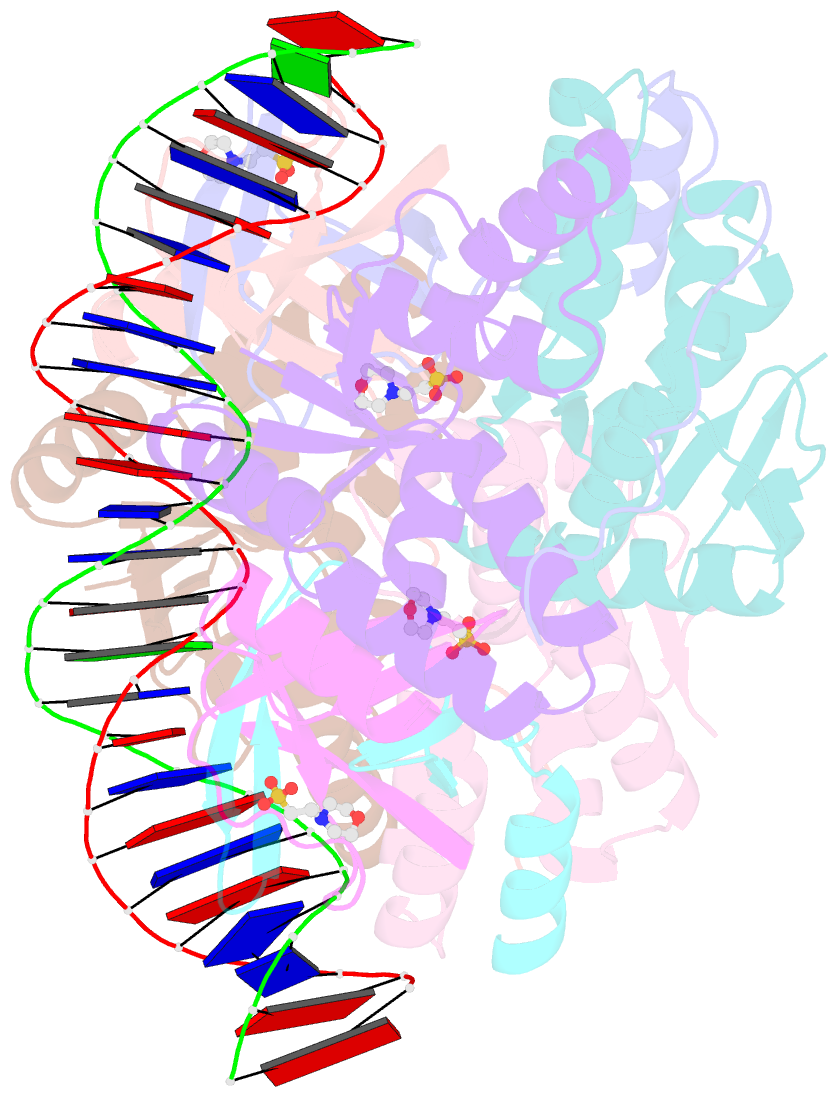
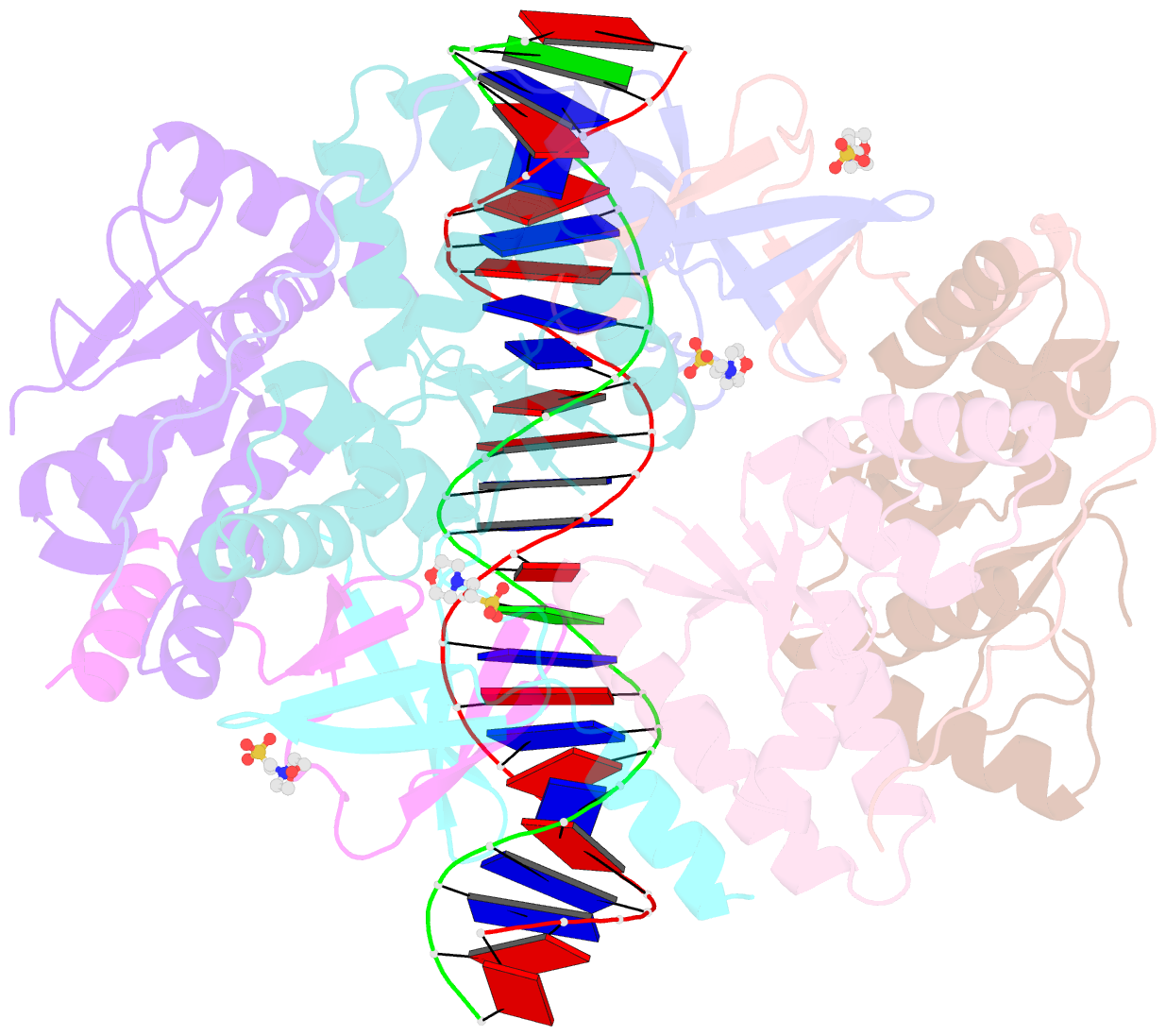
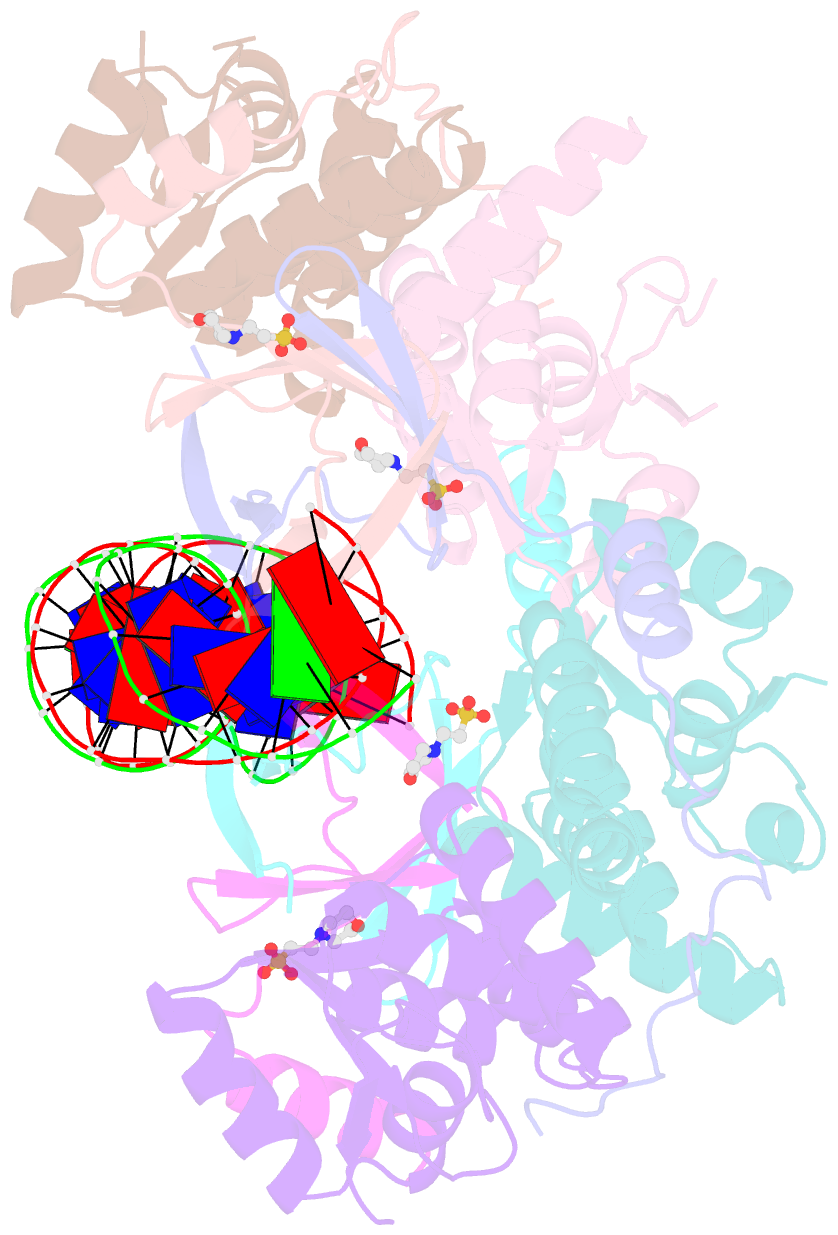
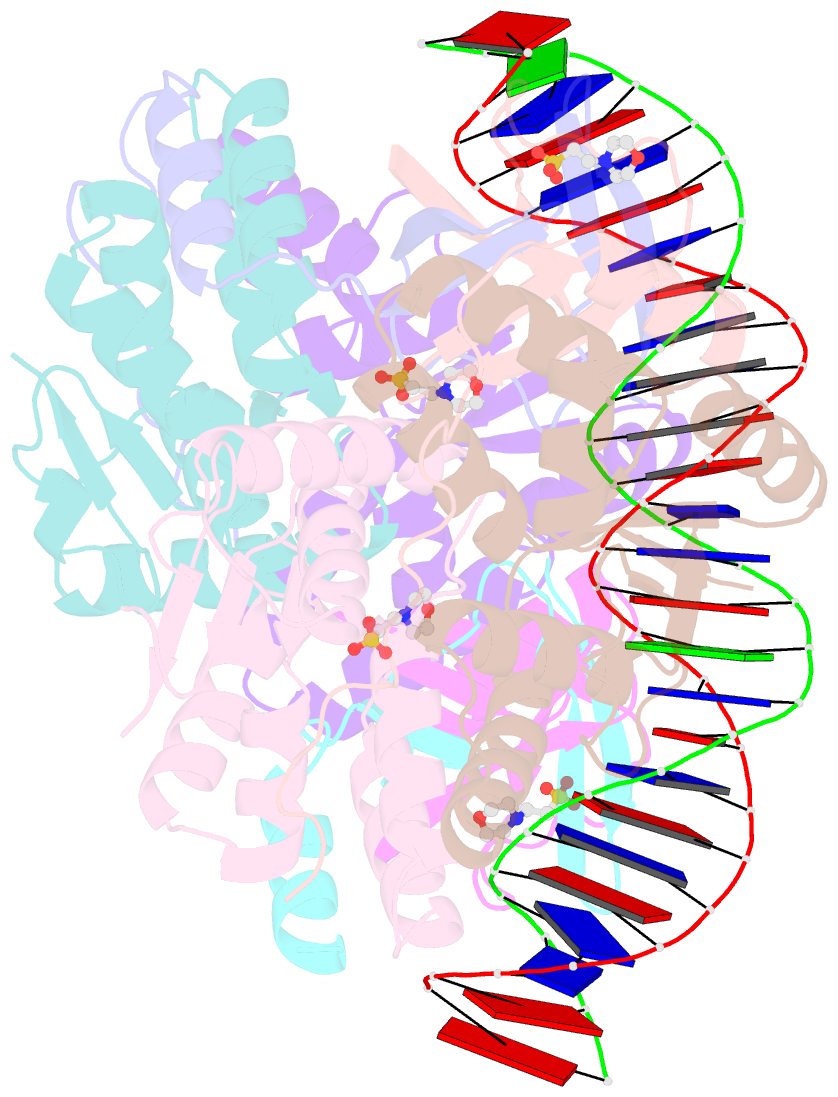
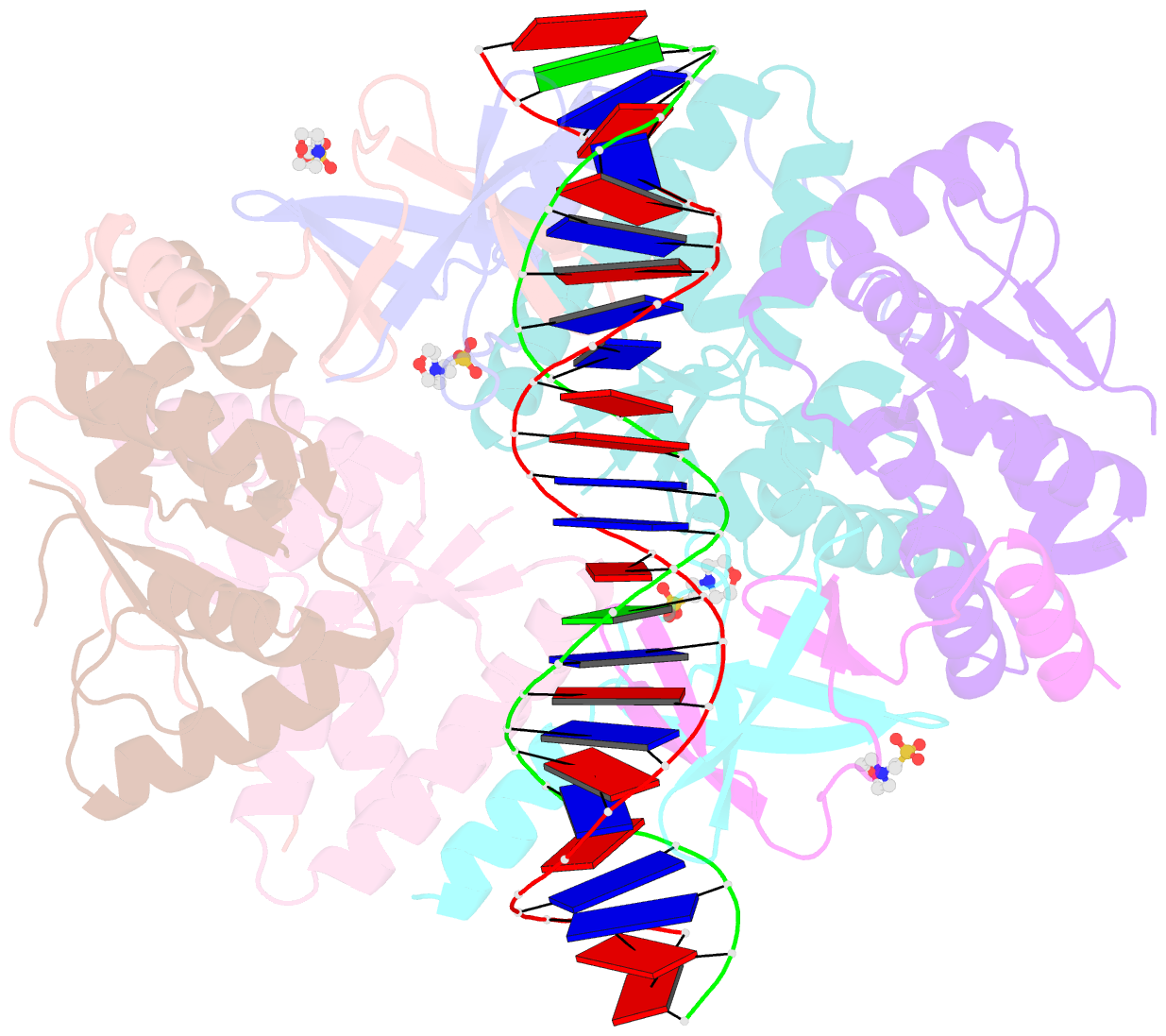
Summary information and primary citation
- PDB-id
- 3zvk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- antitoxin-toxin-DNA
- Method
- X-ray (2.5 Å)
- Summary
- Crystal structure of vapbc2 from rickettsia felis bound to a DNA fragment from their promoter
- Reference
- Mate MJ, Vincentelli R, Foos N, Raoult D, Cambillau C, Ortiz-Lombardia M (2012): "Crystal Structure of the DNA-Bound Vapbc2 Antitoxin/Toxin Pair from Rickettsia Felis." Nucleic Acids Res., 40, 3245. doi: 10.1093/NAR/GKR1167.
- Abstract
- Besides their commonly attributed role in the maintenance of low-copy number plasmids, toxin/antitoxin (TA) loci, also called 'addiction modules', have been found in chromosomes and associated to a number of biological functions such as: reduction of protein synthesis, gene regulation and retardation of cell growth under nutritional stress. The recent discovery of TA loci in obligatory intracellular species of the Rickettsia genus has prompted new research to establish whether they work as stress response elements or as addiction systems that might be toxic for the host cell. VapBC2 is a TA locus from R. felis, a pathogen responsible for flea-borne spotted fever in humans. The VapC2 toxin is a PIN-domain protein, whereas the antitoxin, VapB2, belongs to the family of swapped-hairpin β-barrel DNA-binding proteins. We have used a combination of biophysical and structural methods to characterize this new toxin/antitoxin pair. Our results show how VapB2 can block the VapC2 toxin. They provide a first structural description of the interaction between a swapped-hairpin β-barrel protein and DNA. Finally, these results suggest how the VapC2/VapB2 molar ratio can control the self-regulation of the TA locus transcription.