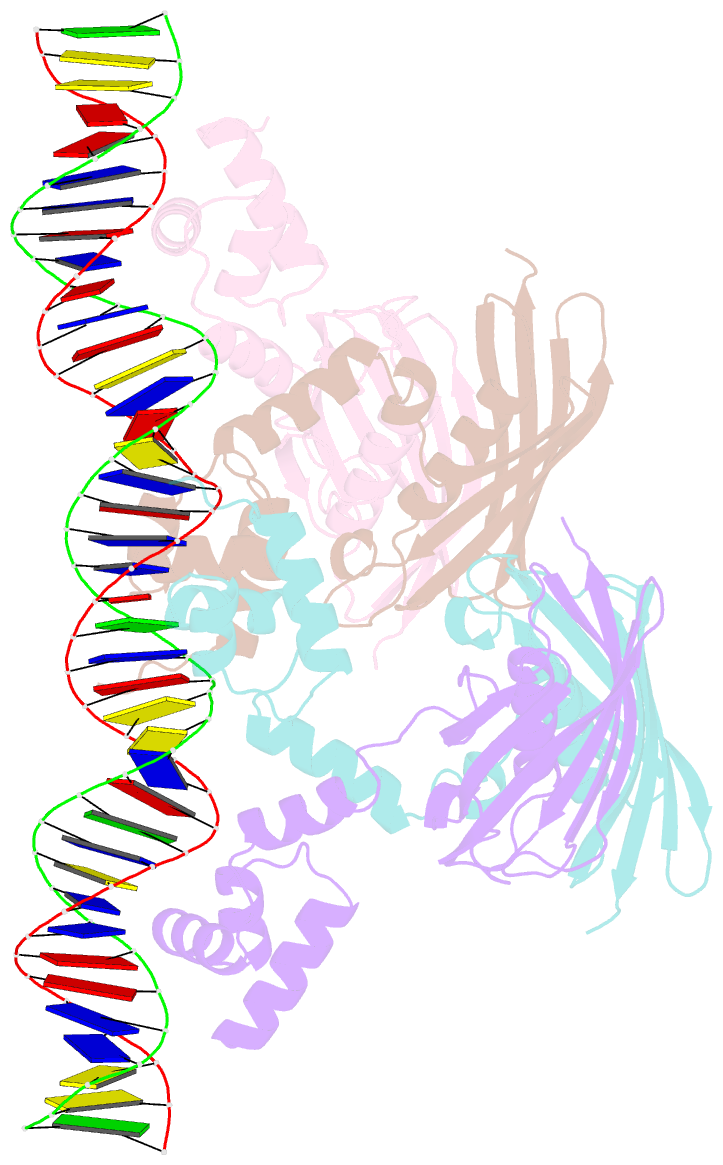
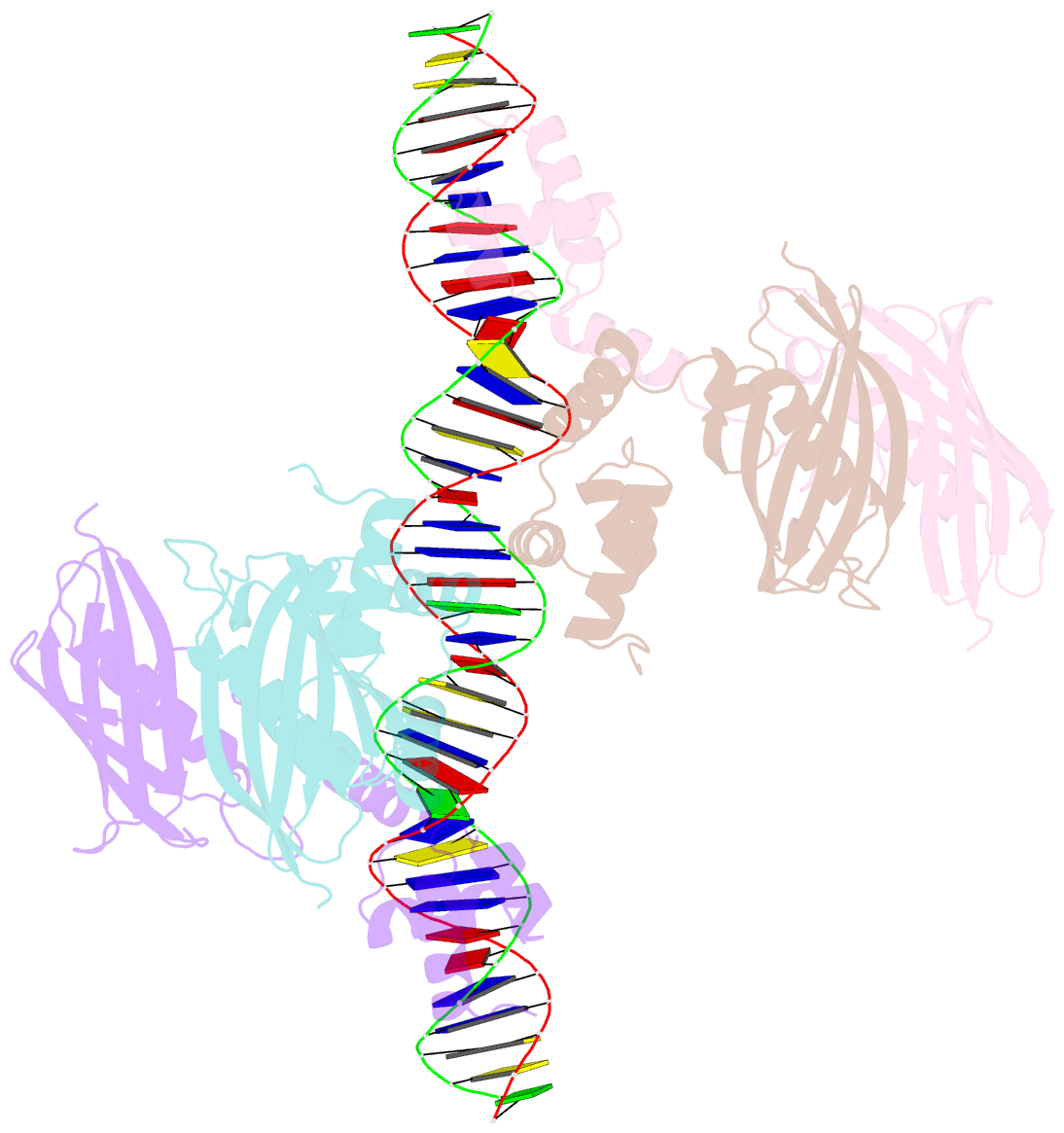
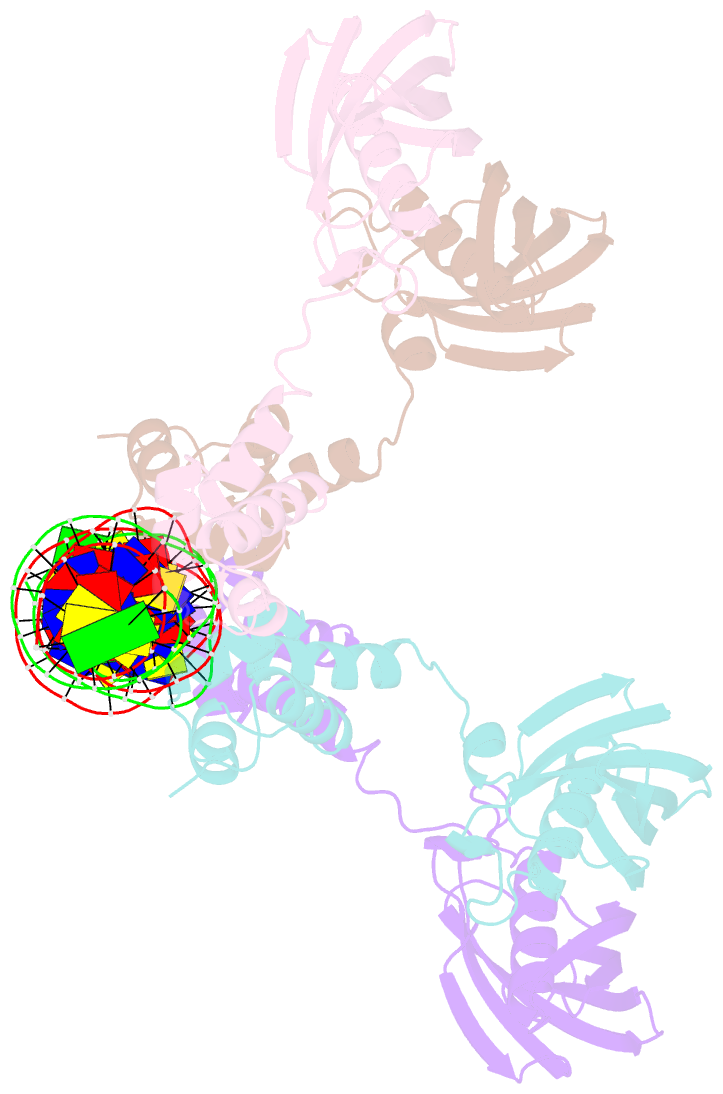
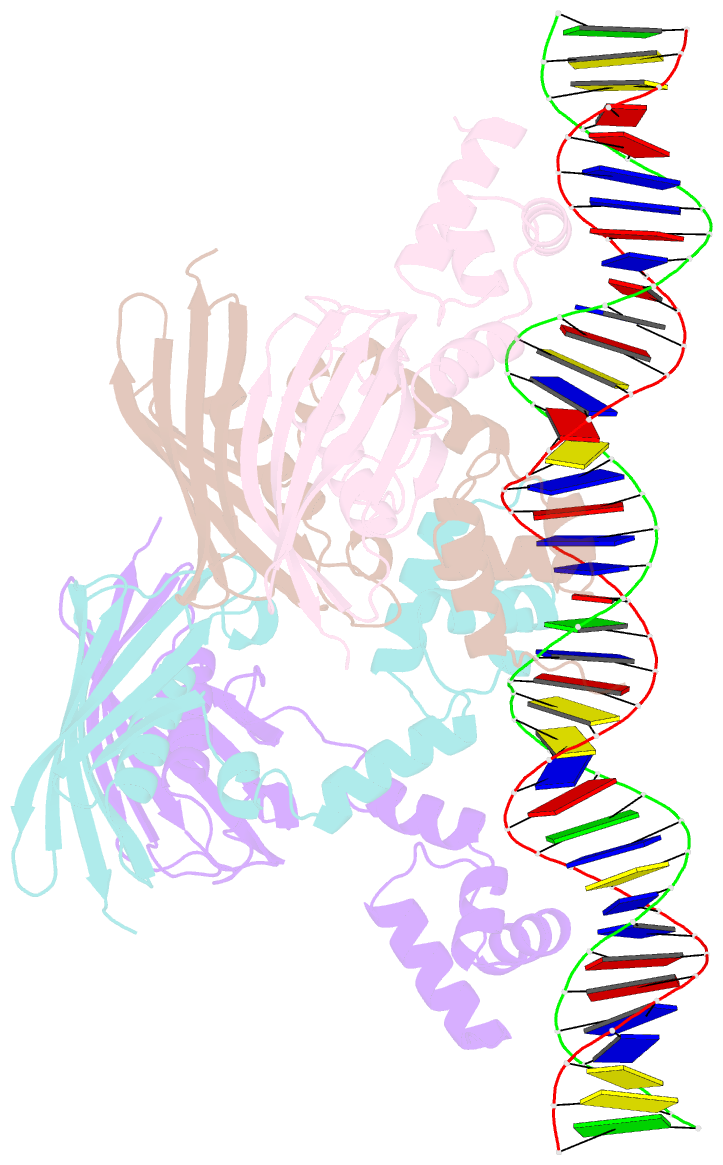
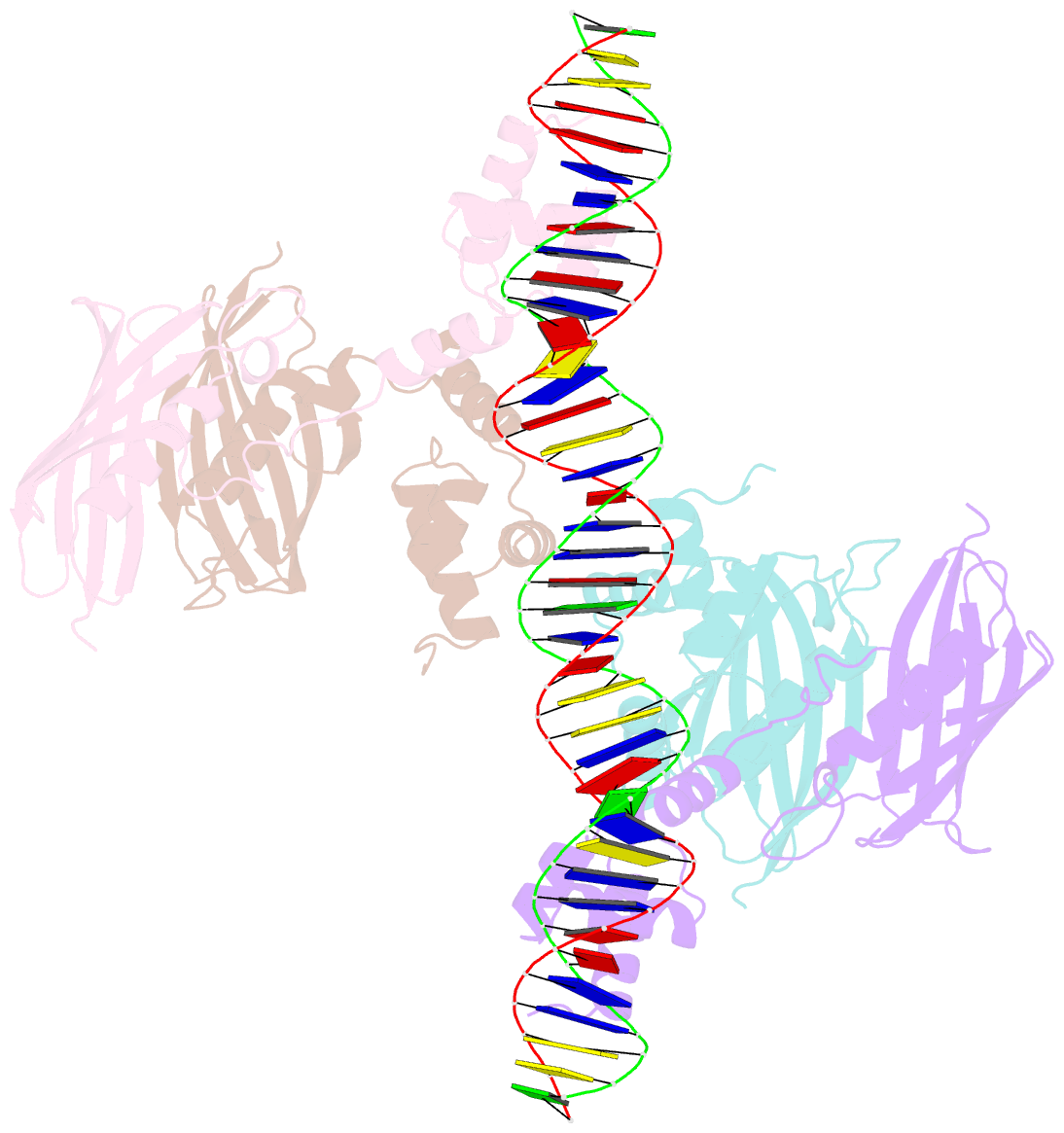
Summary information and primary citation
- PDB-id
- 4a12; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (3.15 Å)
- Summary
- Structure of the global transcription regulator fapr from staphylococcus aureus in complex with DNA operator
- Reference
- Albanesi D, Reh G, Guerin ME, Schaeffer F, Debarbouille M, Buschiazzo A, Schujman GE, De Mendoza D, Alzari PM (2013): "Structural Basis for Feed-Forward Transcriptional Regulation of Membrane Lipid Homeostasis in Staphylococcus Aureus." Plos Pathog., 9, 3108. doi: 10.1371/JOURNAL.PPAT.1003108.
- Abstract
- The biosynthesis of membrane lipids is an essential pathway for virtually all bacteria. Despite its potential importance for the development of novel antibiotics, little is known about the underlying signaling mechanisms that allow bacteria to control their membrane lipid composition within narrow limits. Recent studies disclosed an elaborate feed-forward system that senses the levels of malonyl-CoA and modulates the transcription of genes that mediate fatty acid and phospholipid synthesis in many Gram-positive bacteria including several human pathogens. A key component of this network is FapR, a transcriptional regulator that binds malonyl-CoA, but whose mode of action remains enigmatic. We report here the crystal structures of FapR from Staphylococcus aureus (SaFapR) in three relevant states of its regulation cycle. The repressor-DNA complex reveals that the operator binds two SaFapR homodimers with different affinities, involving sequence-specific contacts from the helix-turn-helix motifs to the major and minor grooves of DNA. In contrast with the elongated conformation observed for the DNA-bound FapR homodimer, binding of malonyl-CoA stabilizes a different, more compact, quaternary arrangement of the repressor, in which the two DNA-binding domains are attached to either side of the central thioesterase-like domain, resulting in a non-productive overall conformation that precludes DNA binding. The structural transition between the DNA-bound and malonyl-CoA-bound states of SaFapR involves substantial changes and large (>30 Å) inter-domain movements; however, both conformational states can be populated by the ligand-free repressor species, as confirmed by the structure of SaFapR in two distinct crystal forms. Disruption of the ability of SaFapR to monitor malonyl-CoA compromises cell growth, revealing the essentiality of membrane lipid homeostasis for S. aureus survival and uncovering novel opportunities for the development of antibiotics against this major human pathogen.