Summary information and primary citation
- PDB-id
- 4am3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (3.0 Å)
- Summary
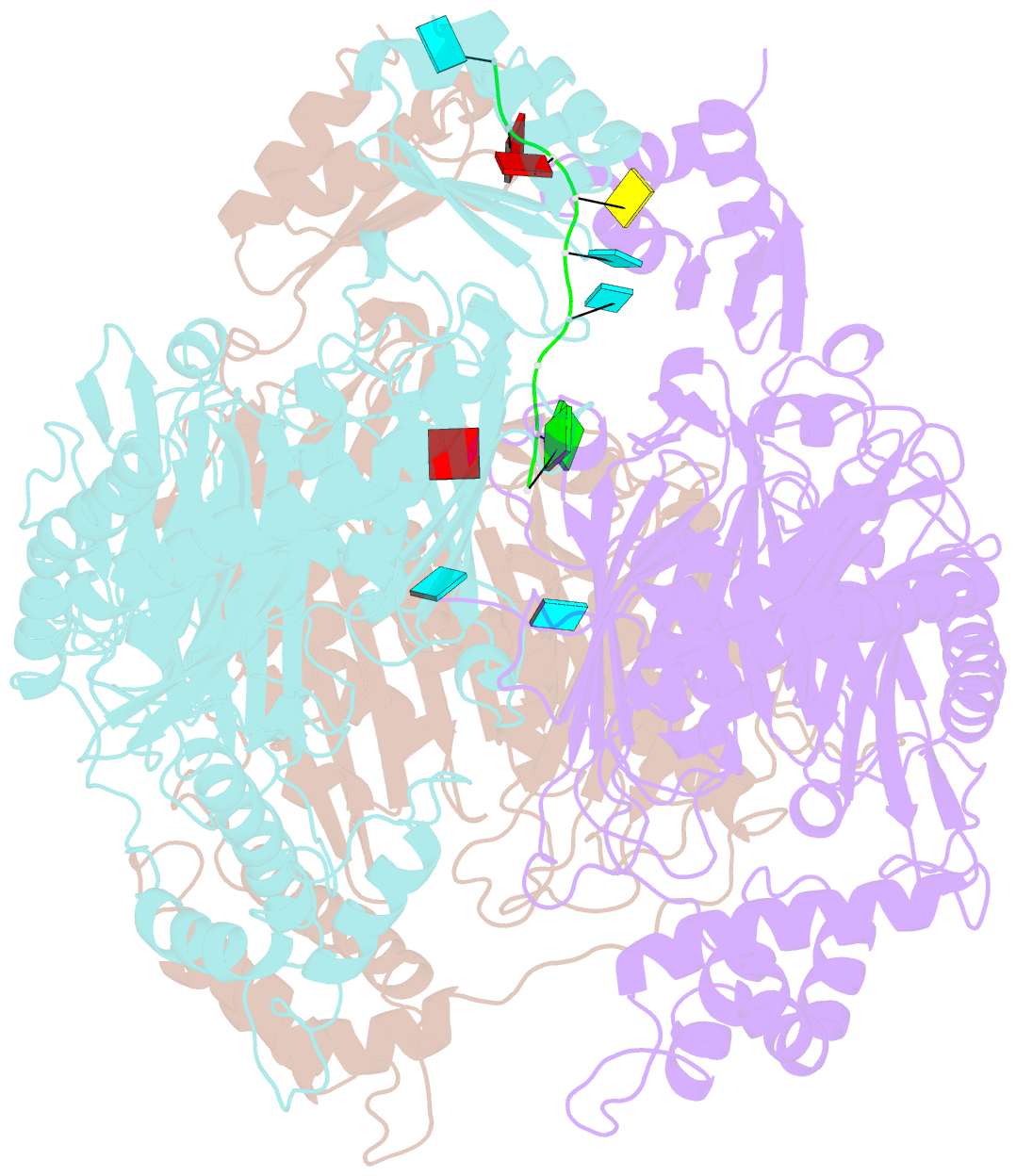
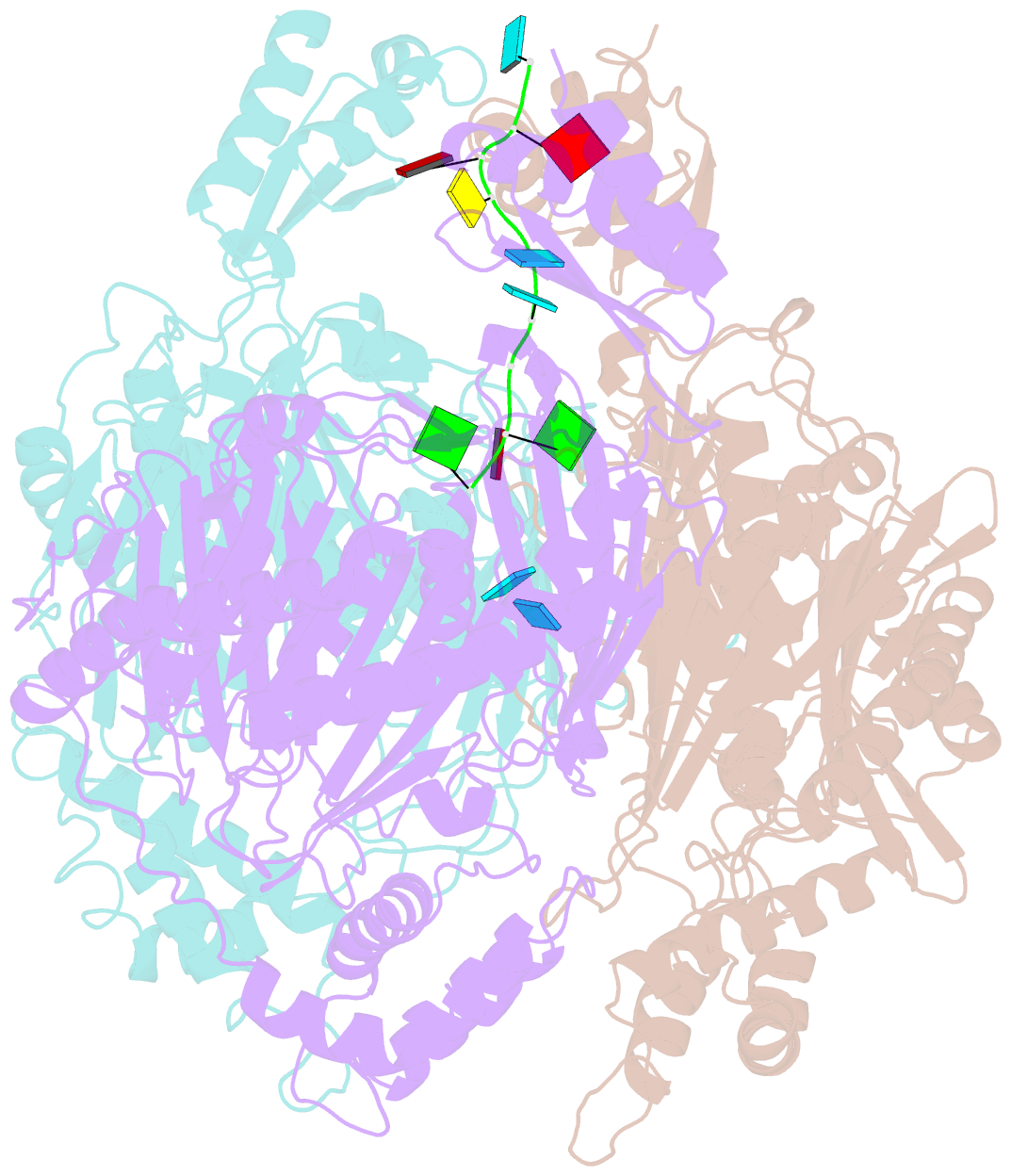
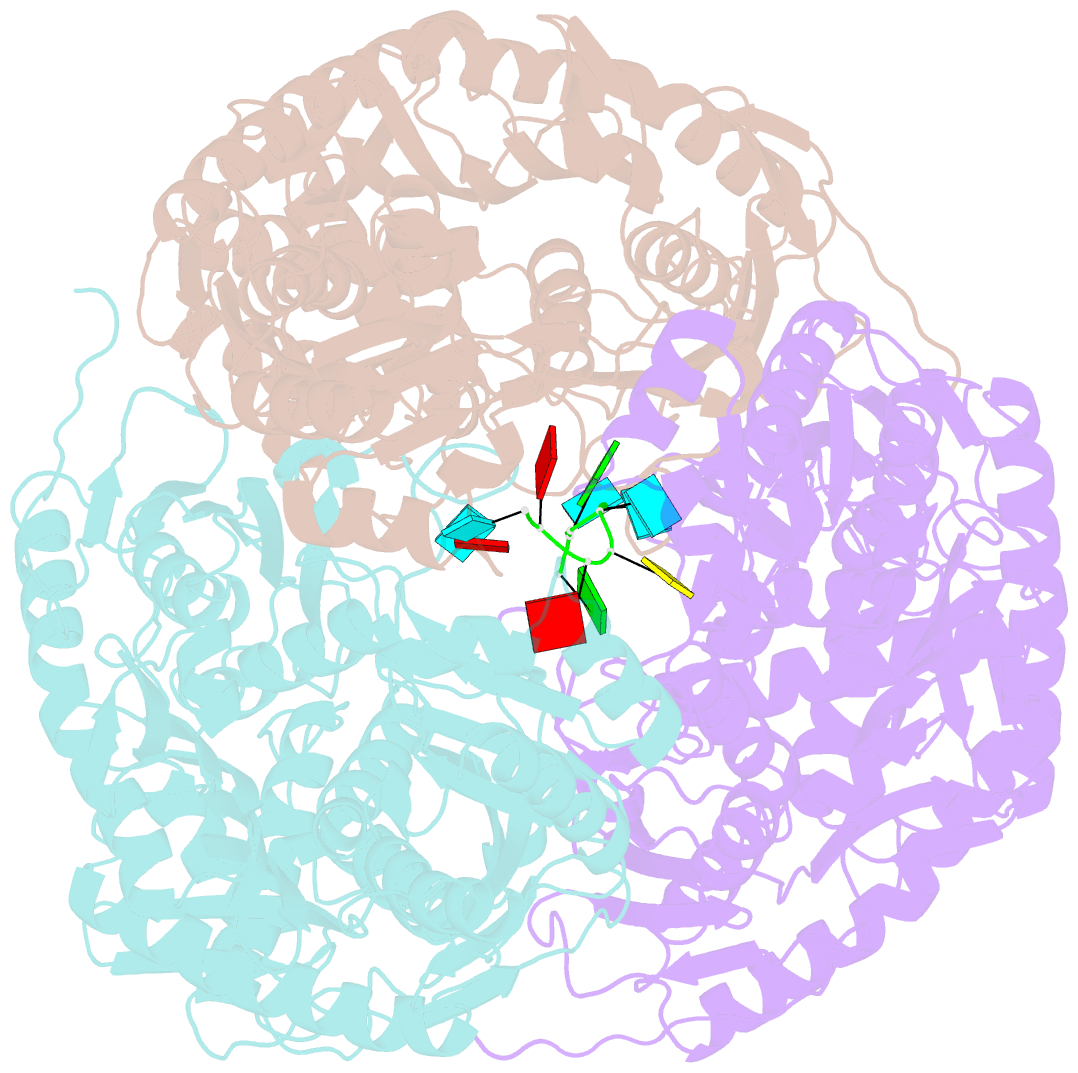
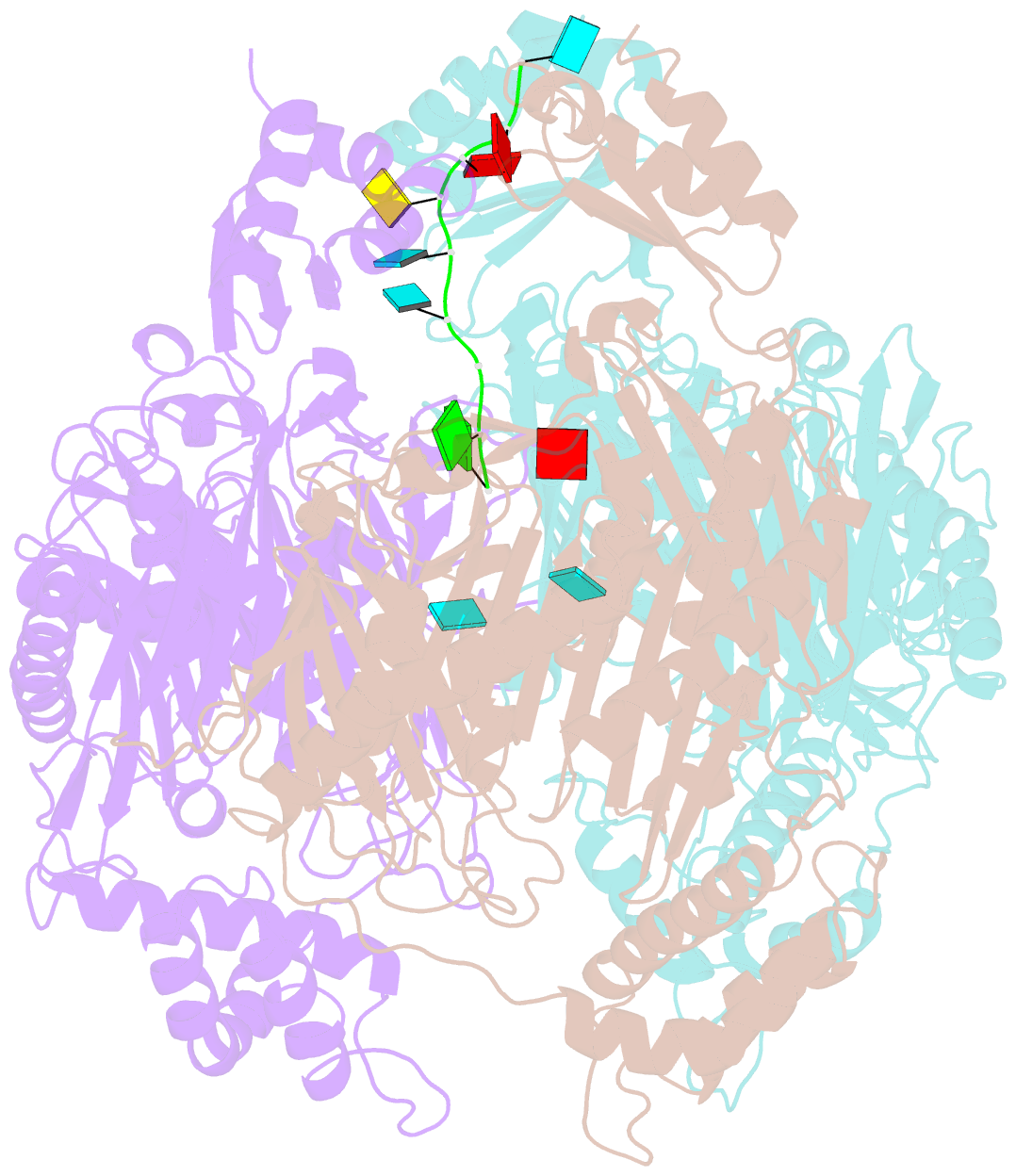
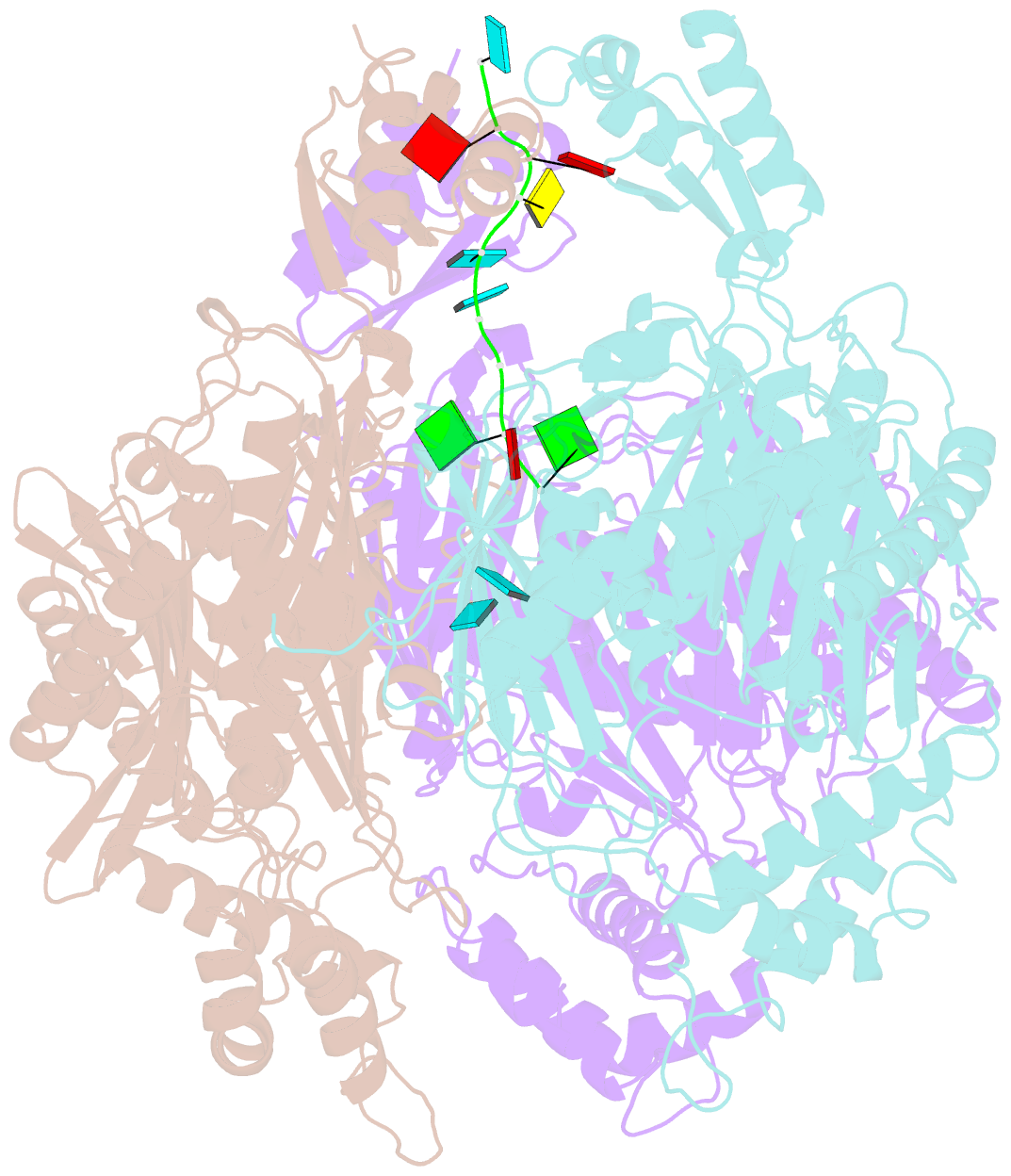
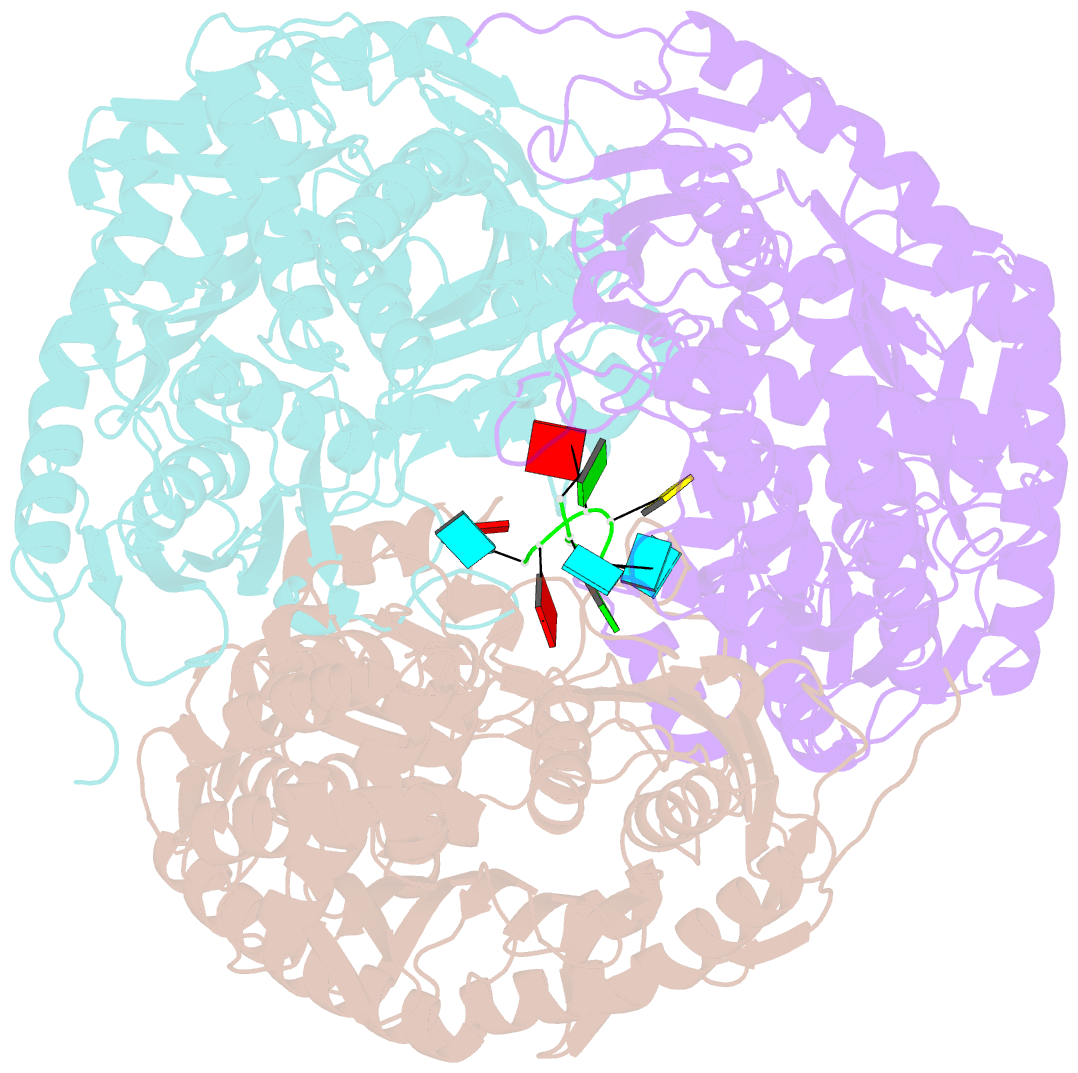
- Crystal structure of c. crescentus pnpase bound to RNA
- Reference
- Hardwick SW, Gubbey T, Hug I, Jenal U, Luisi BF (2012): "Crystal Structure of Caulobacter Crescentus Polynucleotide Phosphorylase Reveals a Mechanism of RNA Substrate Channelling and RNA Degradosome Assembly." Open Biol., 2, 20028. doi: 10.1098/RSOB.120028.
- Abstract
- Polynucleotide phosphorylase (PNPase) is an exoribonuclease that cleaves single-stranded RNA substrates with 3'-5' directionality and processive behaviour. Its ring-like, trimeric architecture creates a central channel where phosphorolytic active sites reside. One face of the ring is decorated with RNA-binding K-homology (KH) and S1 domains, but exactly how these domains help to direct the 3' end of single-stranded RNA substrates towards the active sites is an unsolved puzzle. Insight into this process is provided by our crystal structures of RNA-bound and apo Caulobacter crescentus PNPase. In the RNA-free form, the S1 domains adopt a 'splayed' conformation that may facilitate capture of RNA substrates. In the RNA-bound structure, the three KH domains collectively close upon the RNA and direct the 3' end towards a constricted aperture at the entrance of the central channel. The KH domains make non-equivalent interactions with the RNA, and there is a marked asymmetry within the catalytic core of the enzyme. On the basis of these data, we propose that structural non-equivalence, induced upon RNA binding, helps to channel substrate to the active sites through mechanical ratcheting. Structural and biochemical analyses also reveal the basis for PNPase association with RNase E in the multi-enzyme RNA degradosome assembly of the α-proteobacteria.
Cartoon-block schematics in six views