Summary information and primary citation
- PDB-id
- 4ass; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- structural protein-DNA
- Method
- X-ray (7.0 Å)
- Summary
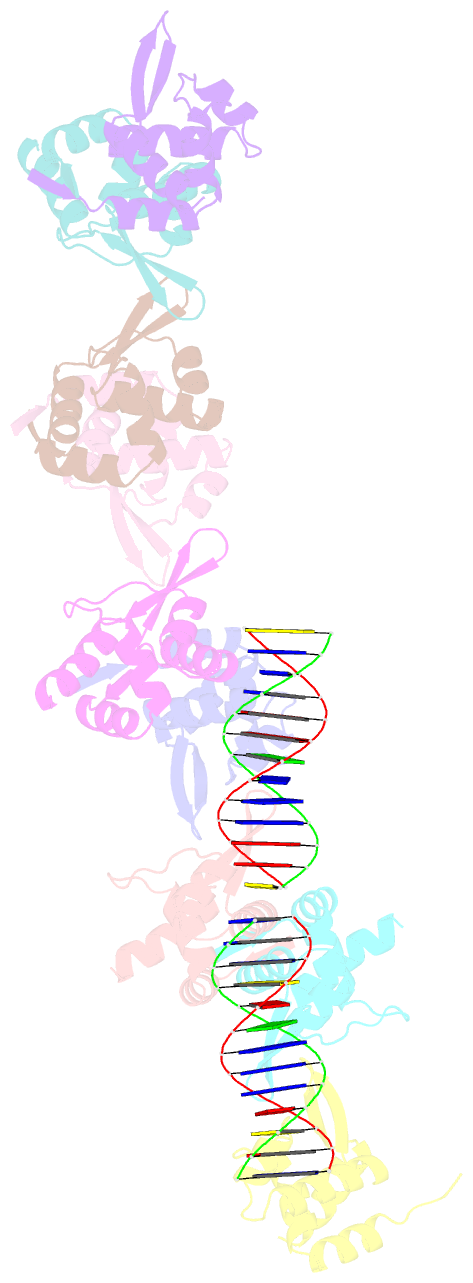
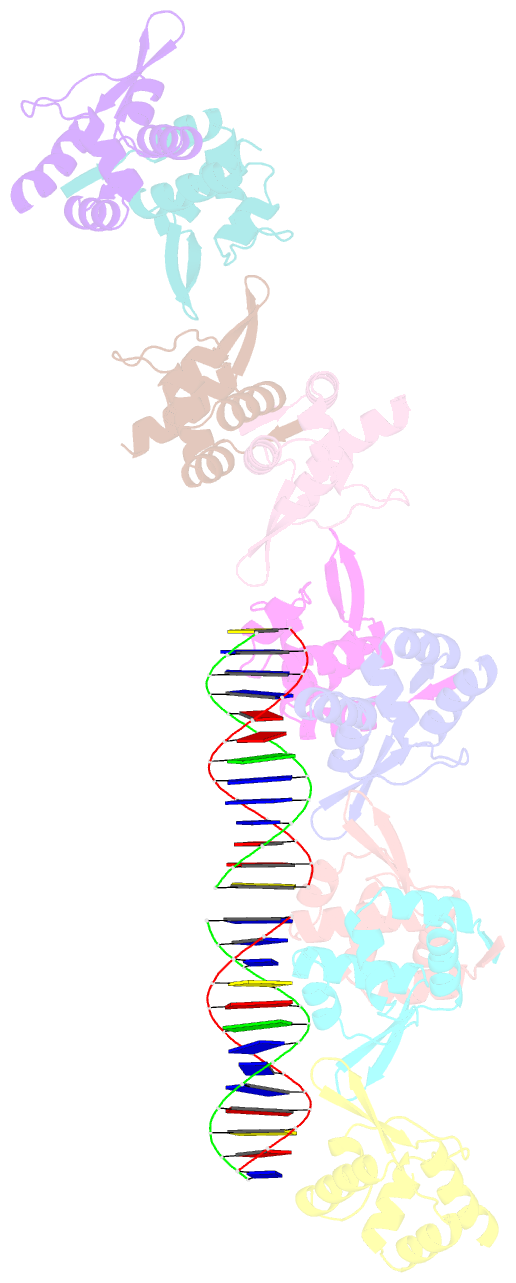
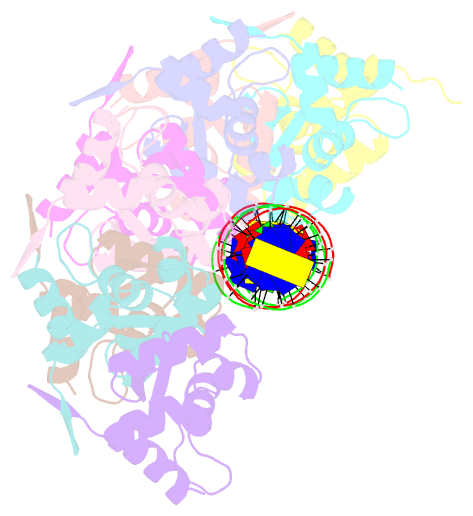
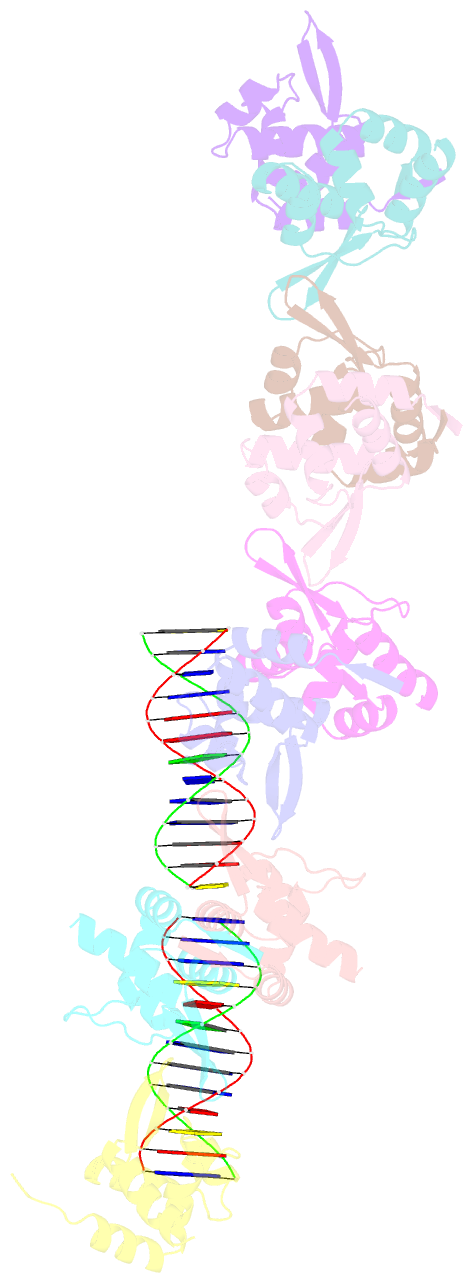
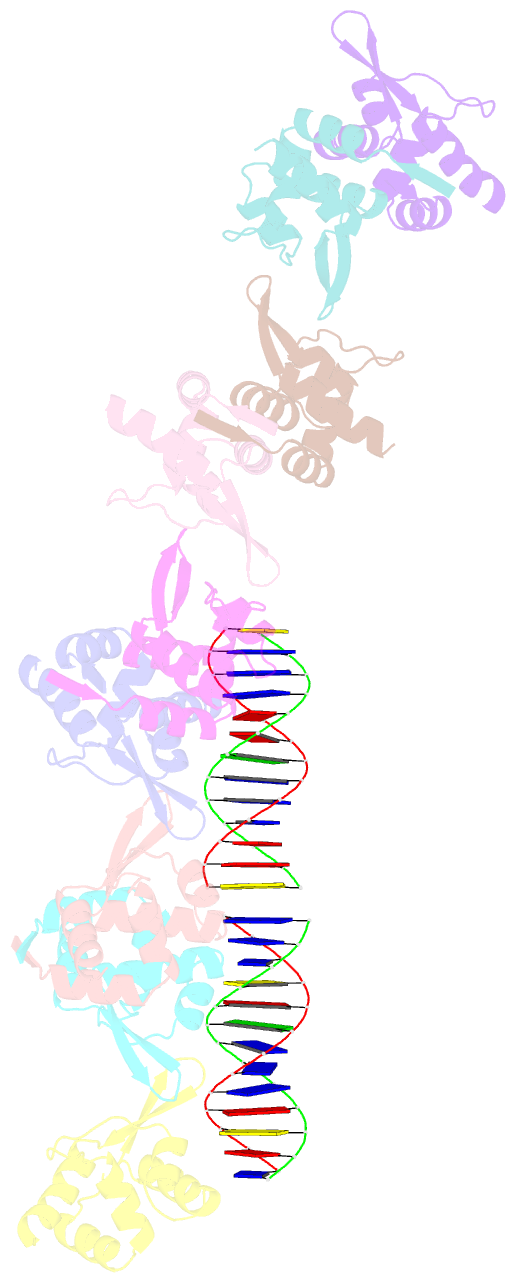
- Tubr bound to tubc - 26 bp - from bacillus thuringiensis serovar israelensis pbtoxis
- Reference
- Aylett CHS, Lowe J (2012): "Superstructure of the Centromeric Complex of Tubzrc Plasmid Partitioning Systems." Proc.Natl.Acad.Sci.USA, 109, 16522. doi: 10.1073/PNAS.1210899109.
- Abstract
- Bacterial plasmid partitioning systems segregate plasmids into each daughter cell. In the well-understood ParMRC plasmid partitioning system, adapter protein ParR binds to centromere parC, forming a helix around which the DNA is externally wrapped. This complex stabilizes the growth of a filament of actin-like ParM protein, which pushes the plasmids to the poles. The TubZRC plasmid partitioning system consists of two proteins, tubulin-like TubZ and TubR, and a DNA centromere, tubC, which perform analogous roles to those in ParMRC, despite being unrelated in sequence and structure. We have dissected in detail the binding sites that comprise Bacillus thuringiensis tubC, visualized the TubRC complex by electron microscopy, and determined a crystal structure of TubR bound to the tubC repeat. We show that the TubRC complex takes the form of a flexible DNA-protein filament, formed by lateral coating along the plasmid from tubC, the full length of which is required for the successful in vitro stabilization of TubZ filaments. We also show that TubR from Bacillus megaterium forms a helical superstructure resembling that of ParR. We suggest that the TubRC DNA-protein filament may bind to, and stabilize, the TubZ filament by forming such a ring-like structure around it. The helical superstructure of this TubRC may indicate convergent evolution between the actin-containing ParMRC and tubulin-containing TubZRC systems.