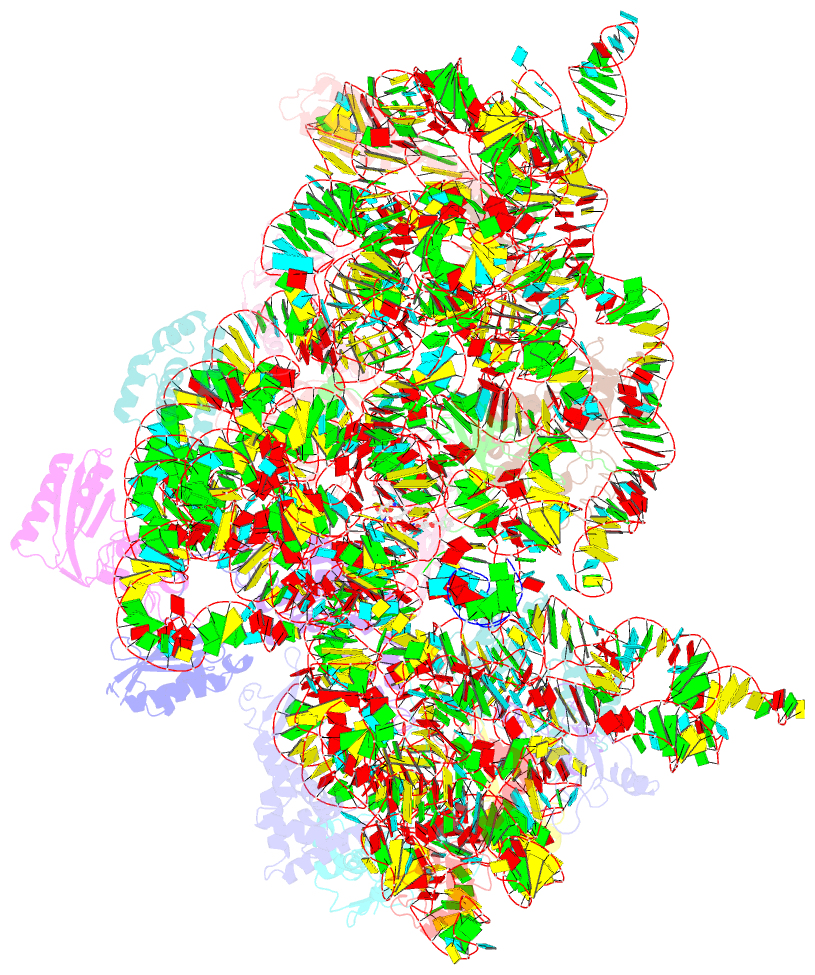
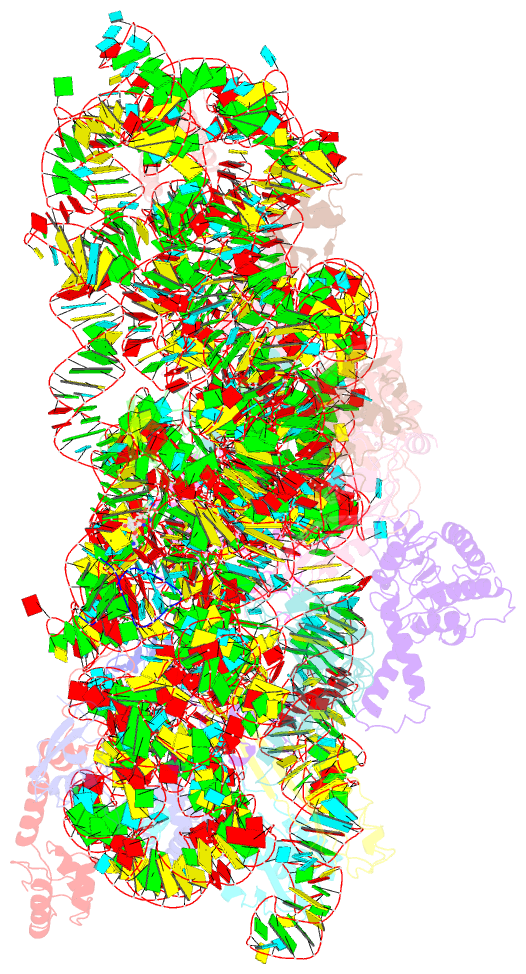
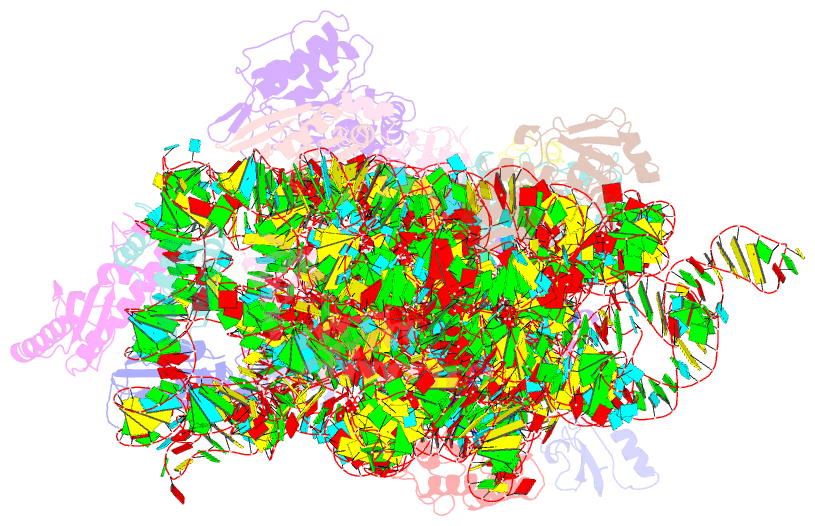
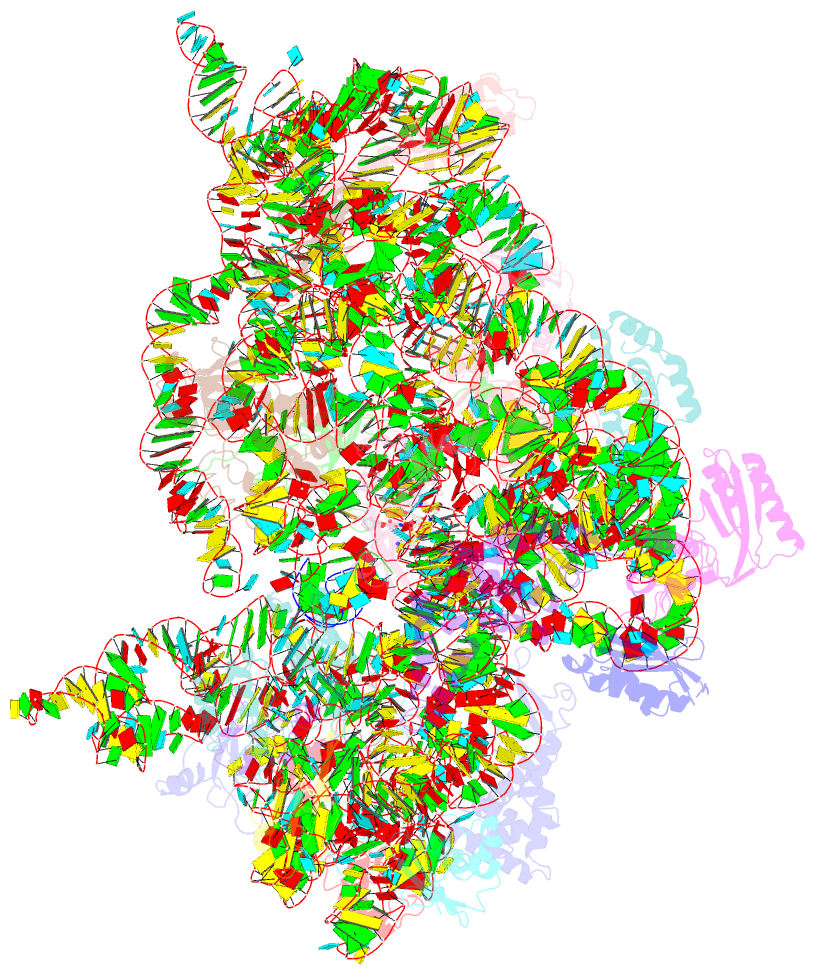
Summary information and primary citation
- PDB-id
- 4b3m; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (2.9 Å)
- Summary
- Crystal structure of the 30s ribosome in complex with compound 1
- Reference
- Perez-Fernandez D, Shcherbakov D, Matt T, Leong NC, Kudyba I, Duscha S, Boukari H, Patak R, Dubakka SR, Lang K, Meyer M, Akbergenov R, Freihofer P, Vaddi S, Thommes P, Ramakrishnan V, Vasella A, Bottger EC (2014): "4'-O-Substitutions Determine Selectivity of Aminoglycoside Antibiotics." Nat.Commun., 5, 3112. doi: 10.1038/NCOMMS4112.
- Abstract
- Clinical use of 2-deoxystreptamine aminoglycoside antibiotics, which target the bacterial ribosome, is compromised by adverse effects related to limited drug selectivity. Here we present a series of 4',6'-O-acetal and 4'-O-ether modifications on glucopyranosyl ring I of aminoglycosides. Chemical modifications were guided by measuring interactions between the compounds synthesized and ribosomes harbouring single point mutations in the drug-binding site, resulting in aminoglycosides that interact poorly with the drug-binding pocket of eukaryotic mitochondrial or cytosolic ribosomes. Yet, these compounds largely retain their inhibitory activity for bacterial ribosomes and show antibacterial activity. Our data indicate that 4'-O-substituted aminoglycosides possess increased selectivity towards bacterial ribosomes and little activity for any of the human drug-binding pockets.