Summary information and primary citation
- PDB-id
- 4cej; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.0 Å)
- Summary
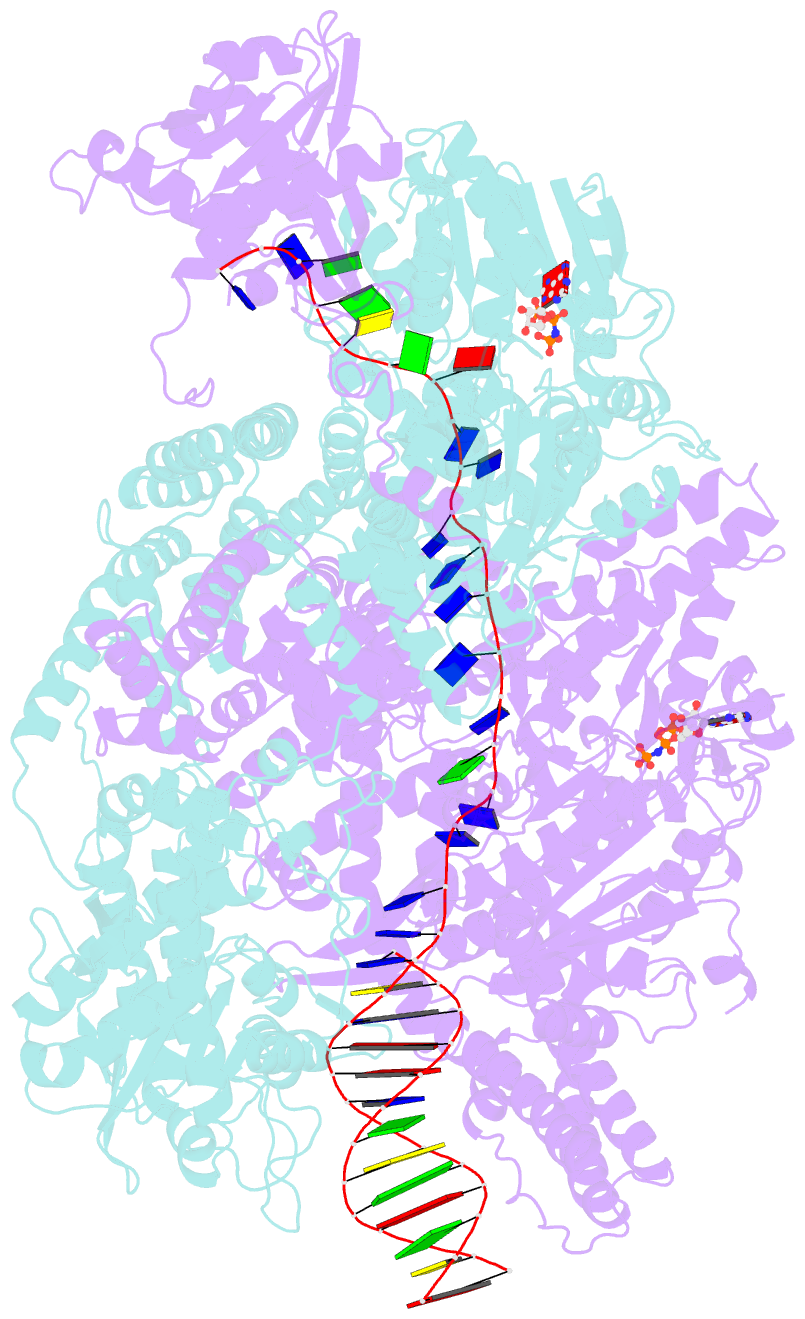
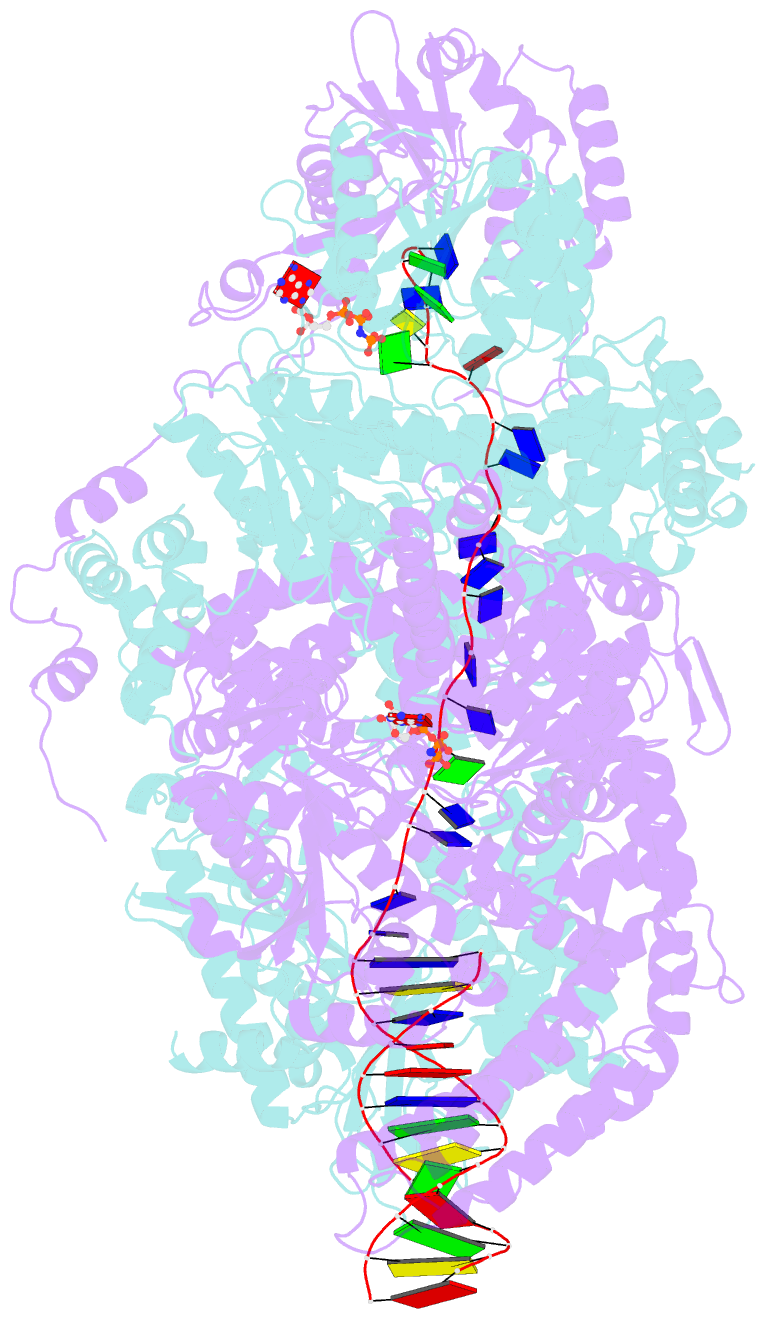
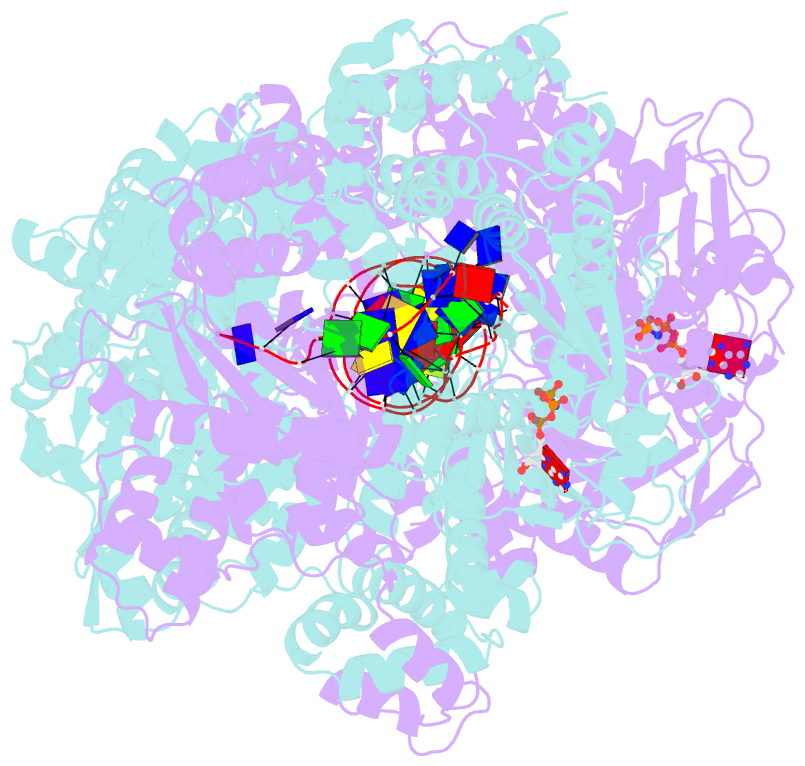
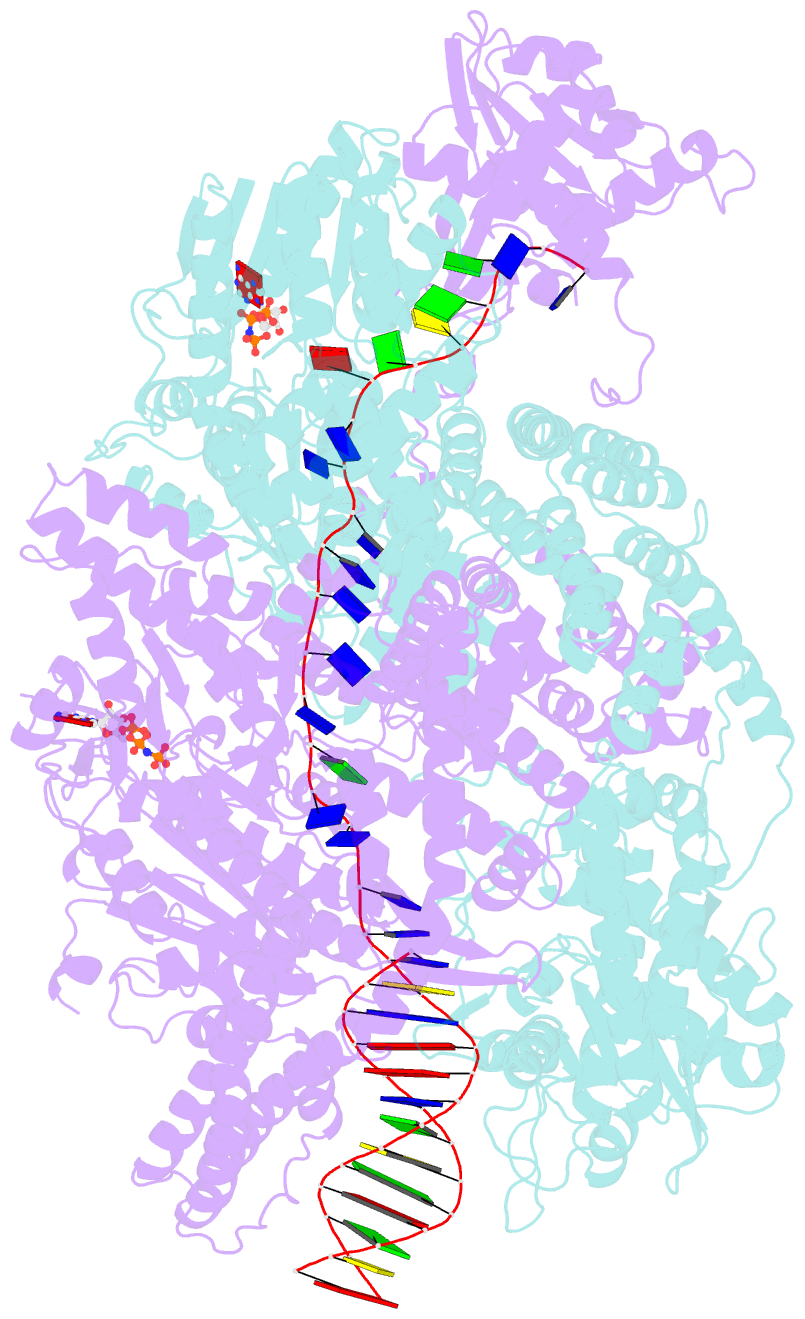
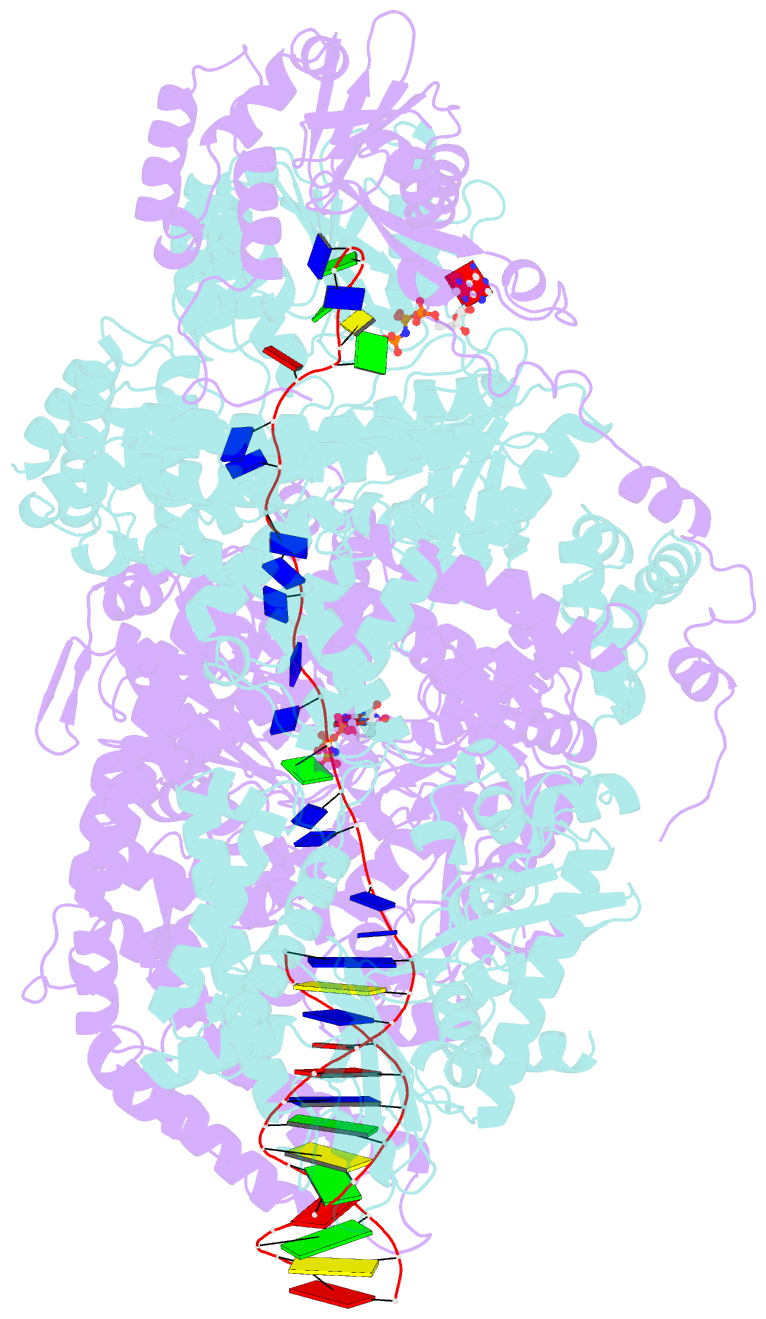
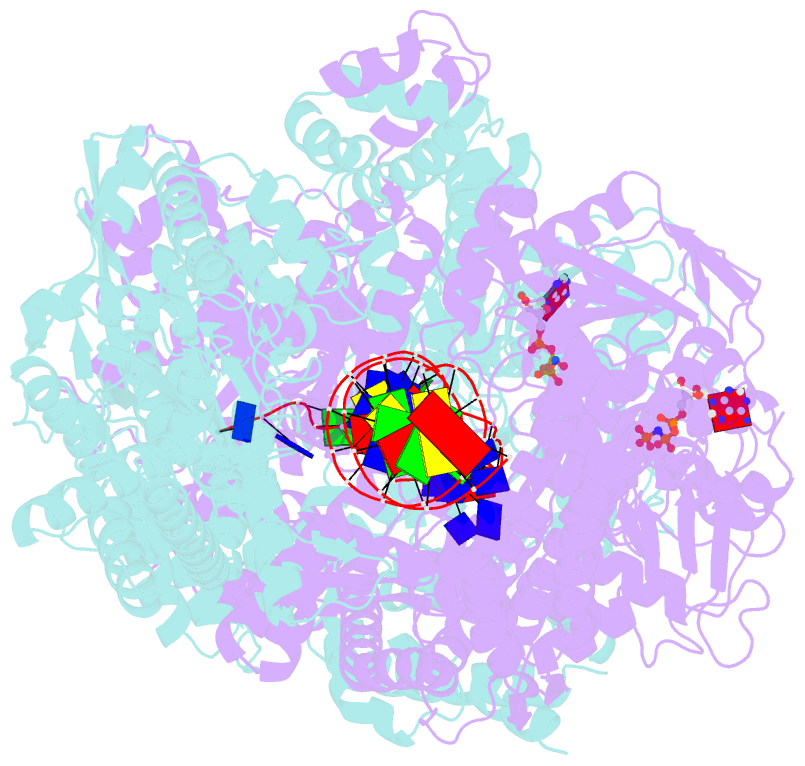
- Crystal structure of addab-DNA-adpnp complex at 3 angstrom resolution
- Reference
- Krajewski WW, Fu X, Wilkinson M, Cronin NB, Dillingham MS, Wigley DB (2014): "Structural Basis for Translocation by Addab Helicase-Nuclease and its Arrest at Chi Sites." Nature, 508, 416. doi: 10.1038/NATURE13037.
- Abstract
- In bacterial cells, processing of double-stranded DNA breaks for repair by homologous recombination is dependent upon the recombination hotspot sequence χ (Chi) and is catalysed by either an AddAB- or RecBCD-type helicase-nuclease (reviewed in refs 3, 4). These enzyme complexes unwind and digest the DNA duplex from the broken end until they encounter a χ sequence, whereupon they produce a 3' single-stranded DNA tail onto which they initiate loading of the RecA protein. Consequently, regulation of the AddAB/RecBCD complex by χ is a key control point in DNA repair and other processes involving genetic recombination. Here we report crystal structures of Bacillus subtilis AddAB in complex with different χ-containing DNA substrates either with or without a non-hydrolysable ATP analogue. Comparison of these structures suggests a mechanism for DNA translocation and unwinding, suggests how the enzyme binds specifically to χ sequences, and explains how χ recognition leads to the arrest of AddAB (and RecBCD) translocation that is observed in single-molecule experiments.