Summary information and primary citation
- PDB-id
- 4csa; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-DNA
- Method
- X-ray (2.28 Å)
- Summary
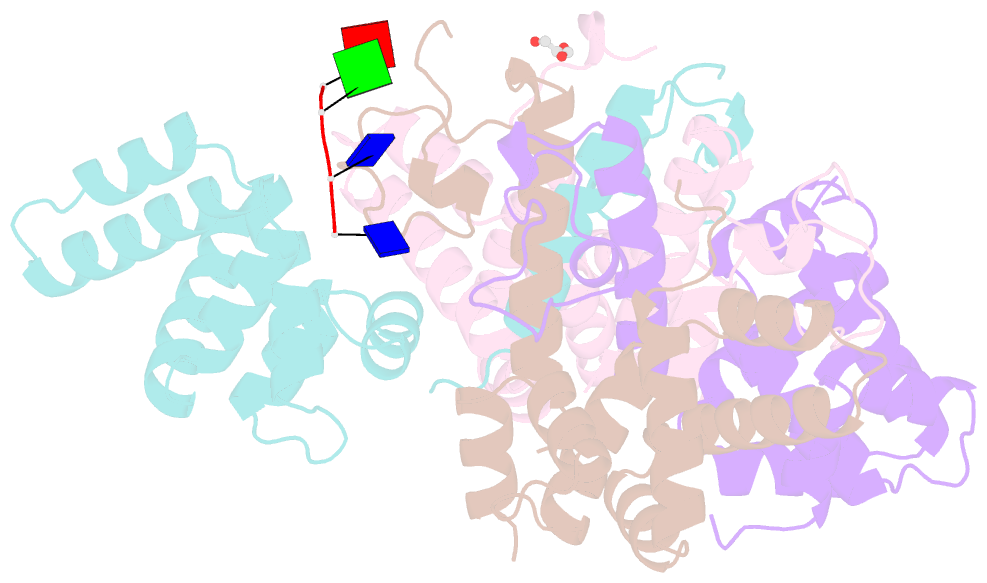
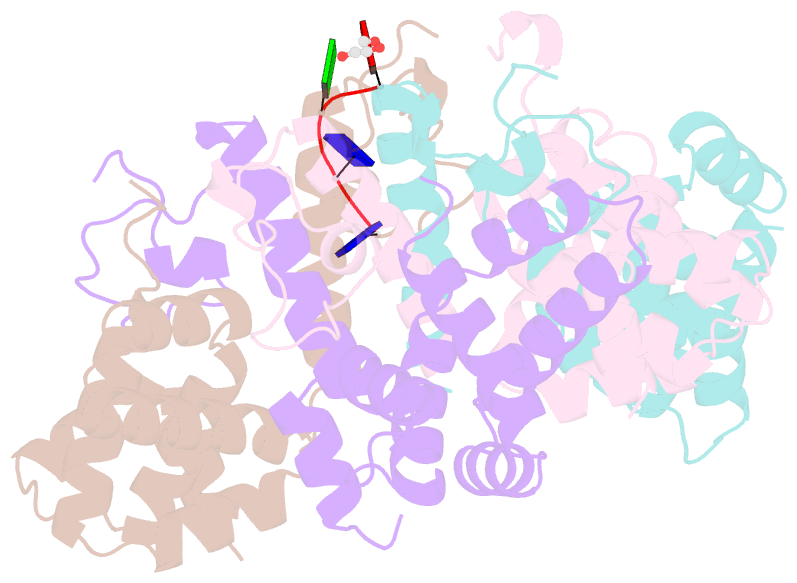
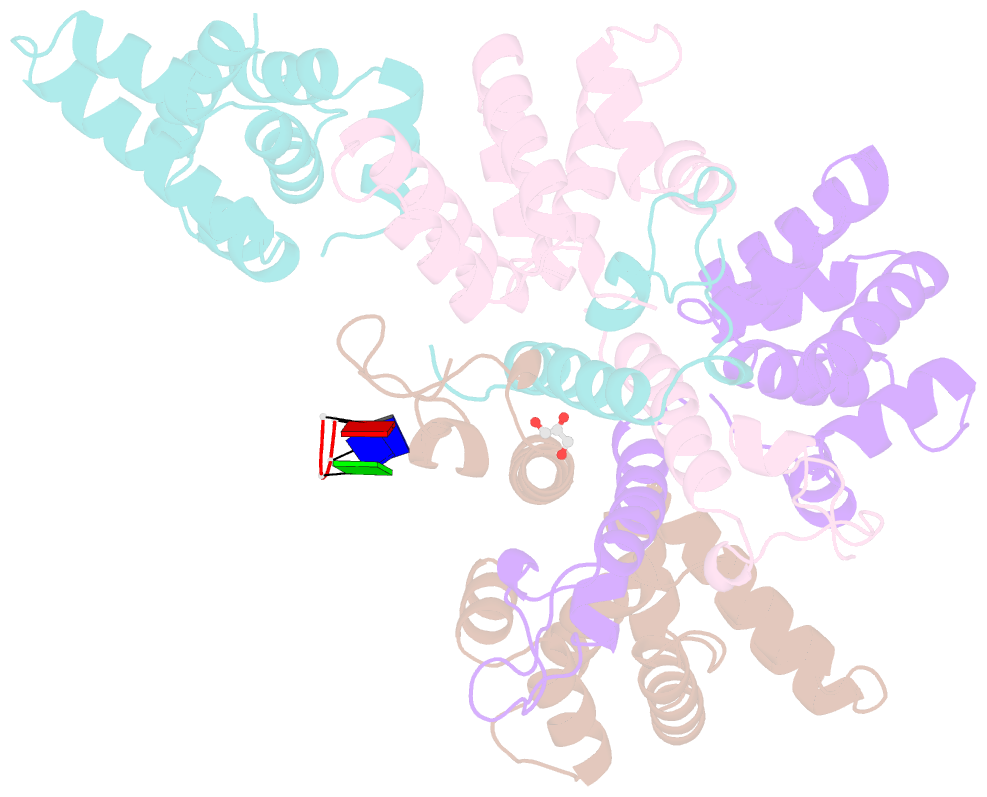
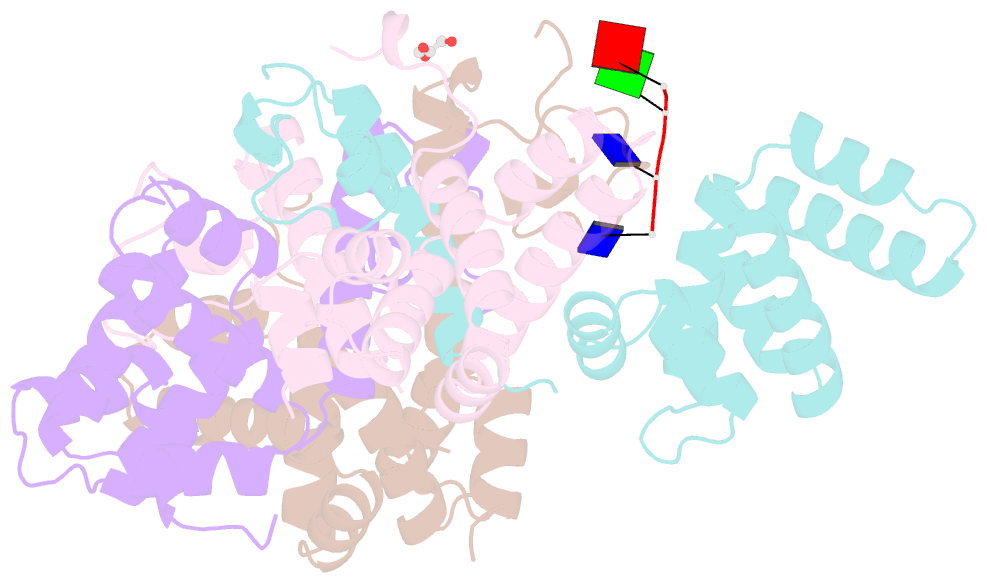
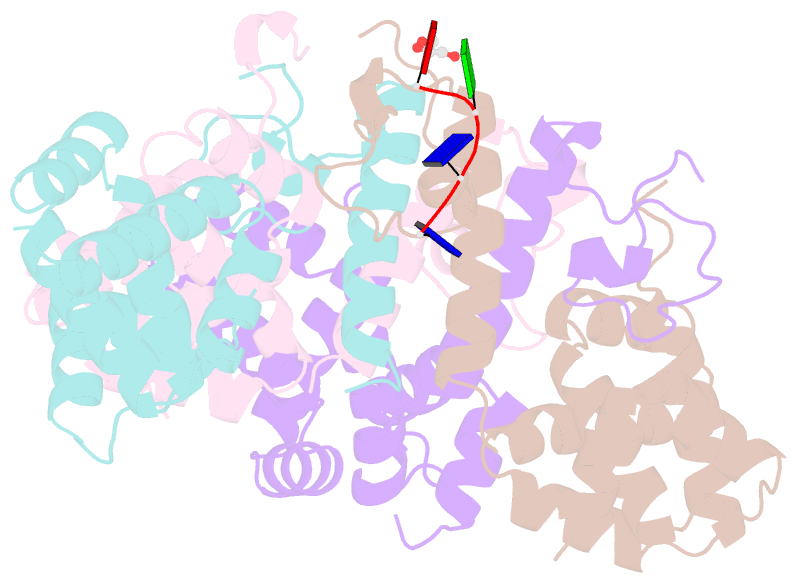
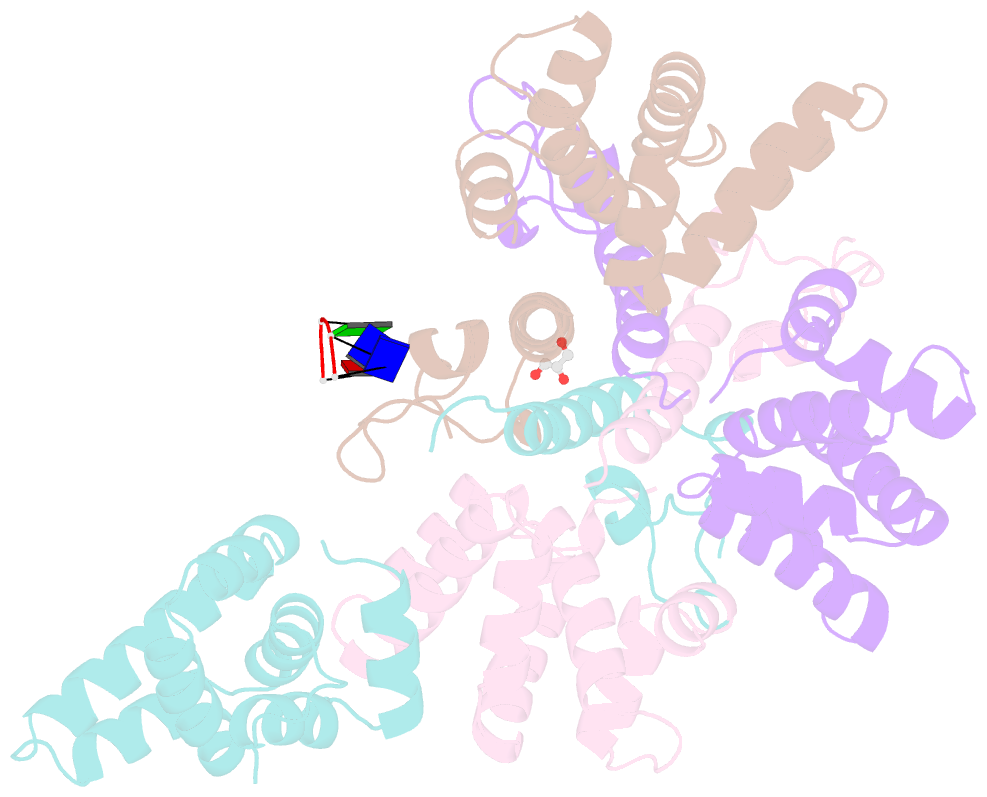
- Crystal structure of the asymmetric human metapneumovirus m2-1 tetramer bound to a DNA 4-mer
- Reference
- Leyrat C, Renner M, Harlos K, Huiskonen JT, Grimes JM (2014): "Drastic Changes in Conformational Dynamics of the Antiterminator M2-1 Regulate Transcription Efficiency in Pneumovirinae." Elife, 3, 02674. doi: 10.7554/ELIFE.02674.
- Abstract
- The M2-1 protein of human metapneumovirus (HMPV) is a zinc-binding transcription antiterminator which is highly conserved among pneumoviruses. We report the structure of tetrameric HMPV M2-1. Each protomer features a N-terminal zinc finger domain and an α-helical tetramerization motif forming a rigid unit, followed by a flexible linker and an α-helical core domain. The tetramer is asymmetric, three of the protomers exhibiting a closed conformation, and one an open conformation. Molecular dynamics simulations and SAXS demonstrate a dynamic equilibrium between open and closed conformations in solution. Structures of adenosine monophosphate- and DNA- bound M2-1 establish the role of the zinc finger domain in base-specific recognition of RNA. Binding to 'gene end' RNA sequences stabilized the closed conformation of M2-1 leading to a drastic shift in the conformational landscape of M2-1. We propose a model for recognition of gene end signals and discuss the implications of these findings for transcriptional regulation in pneumoviruses.DOI: http://dx.doi.org/10.7554/eLife.02674.001.