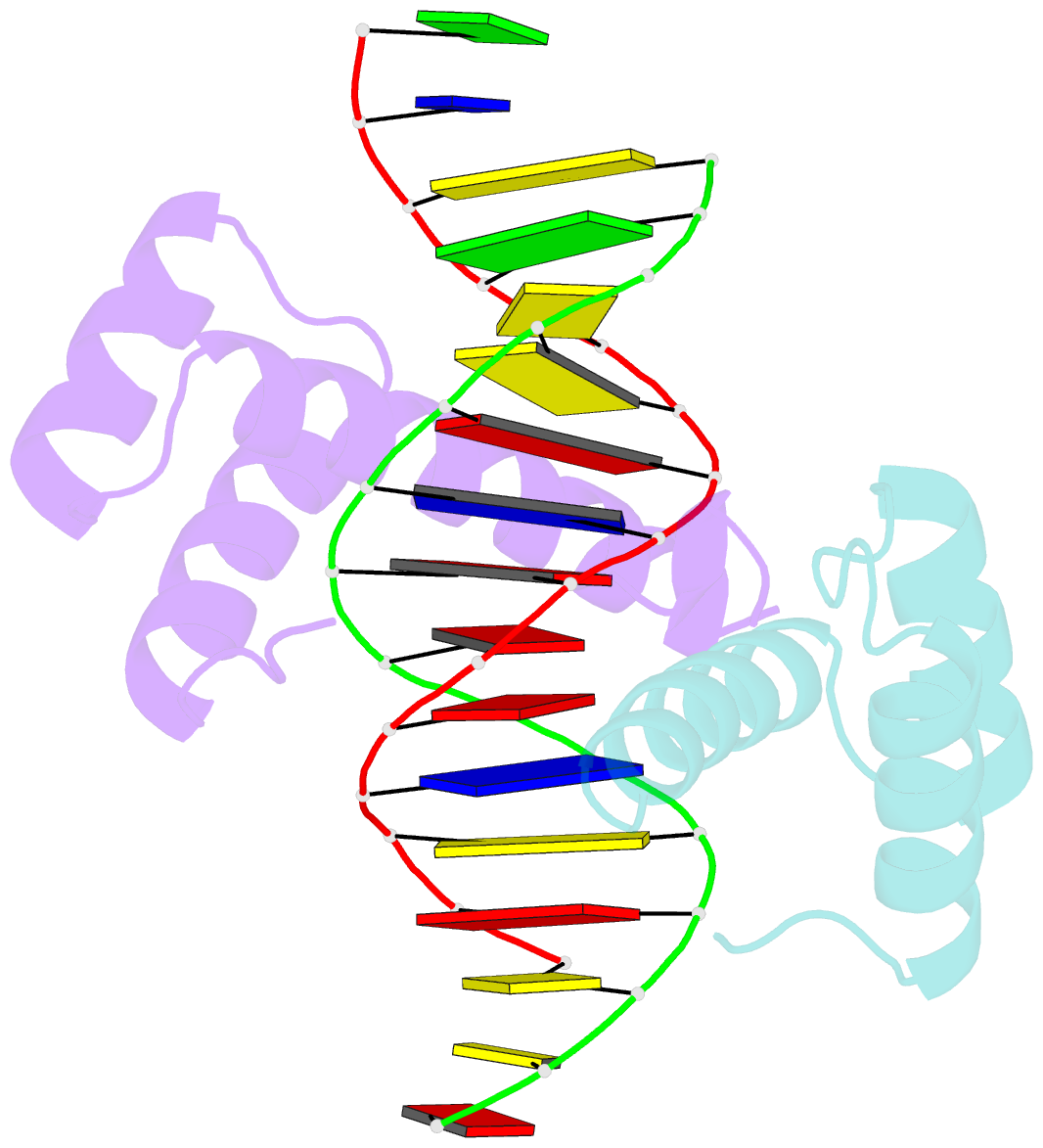
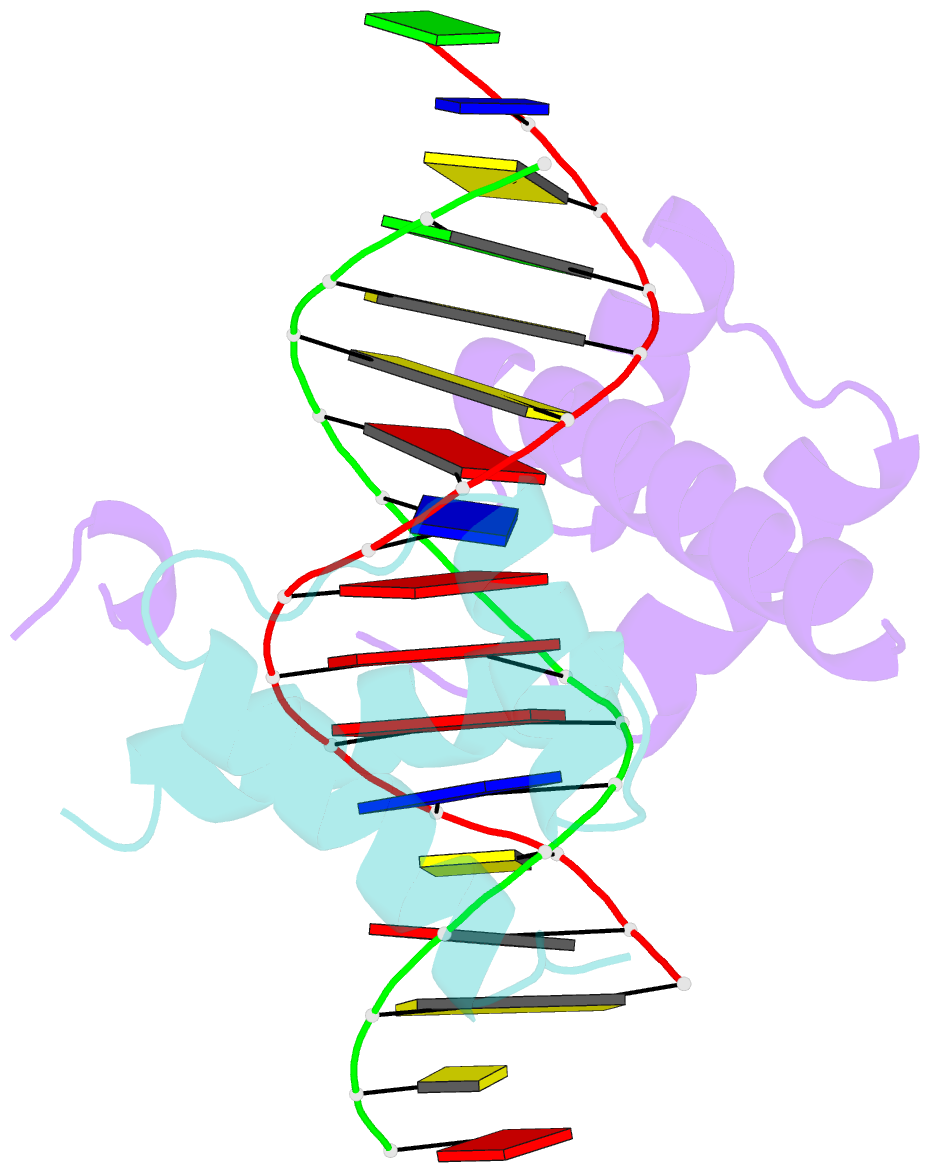
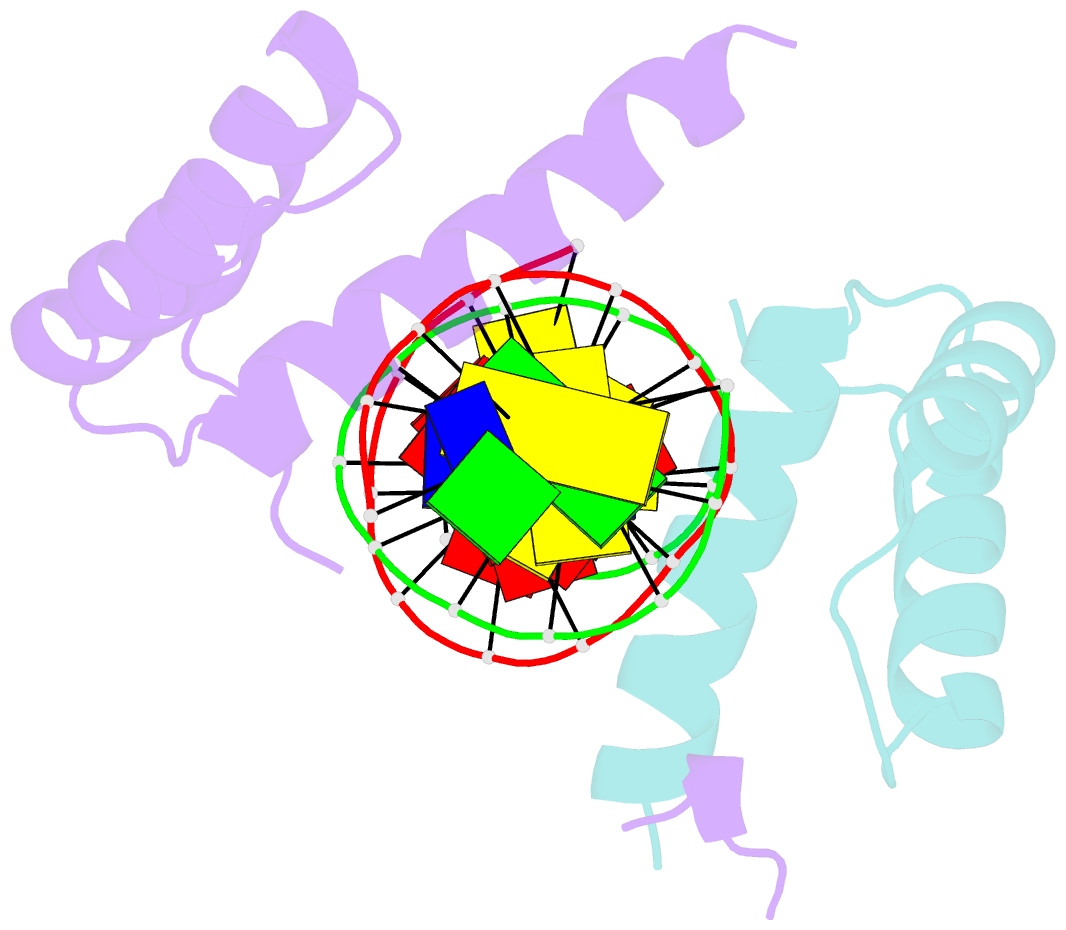
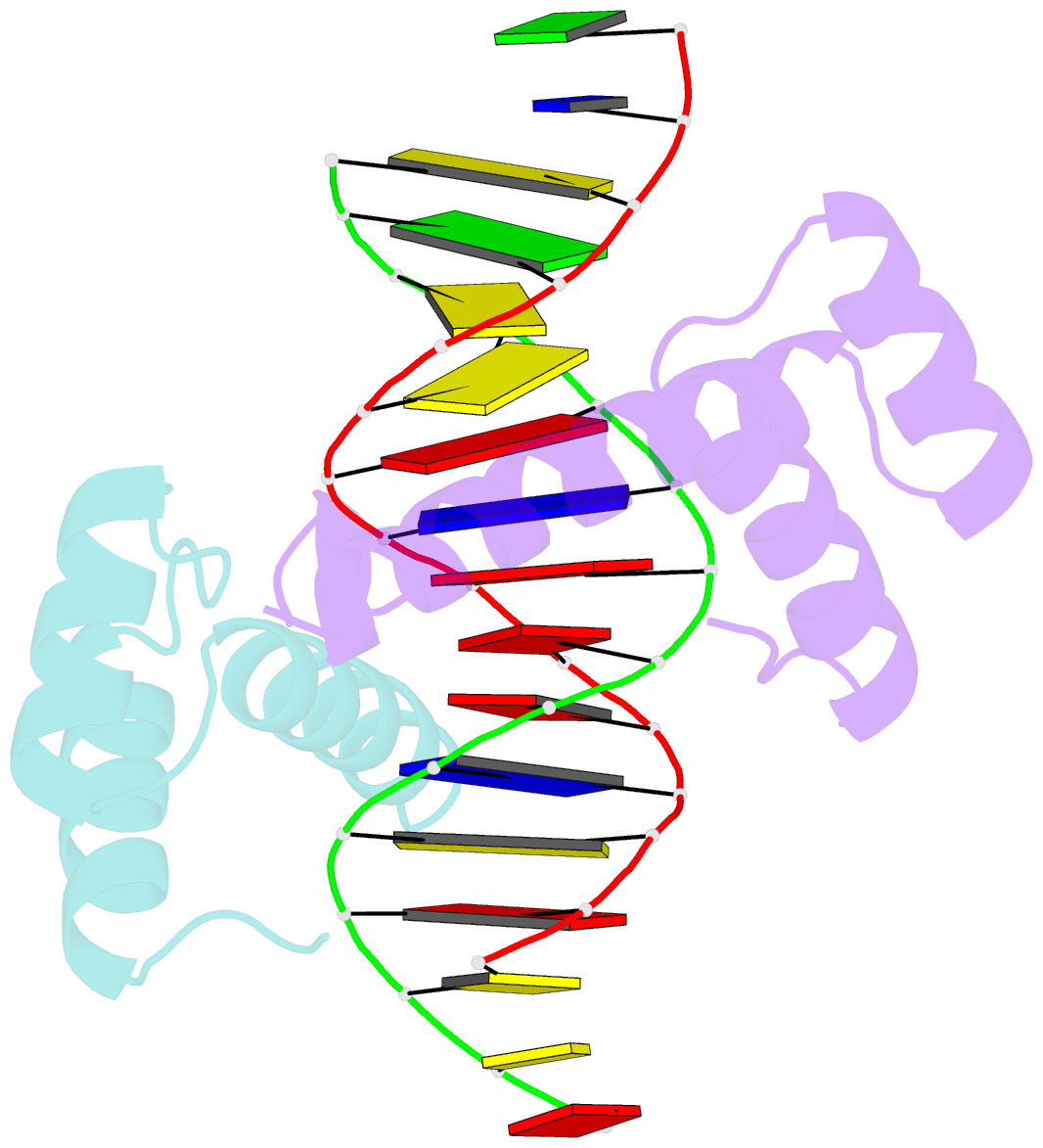
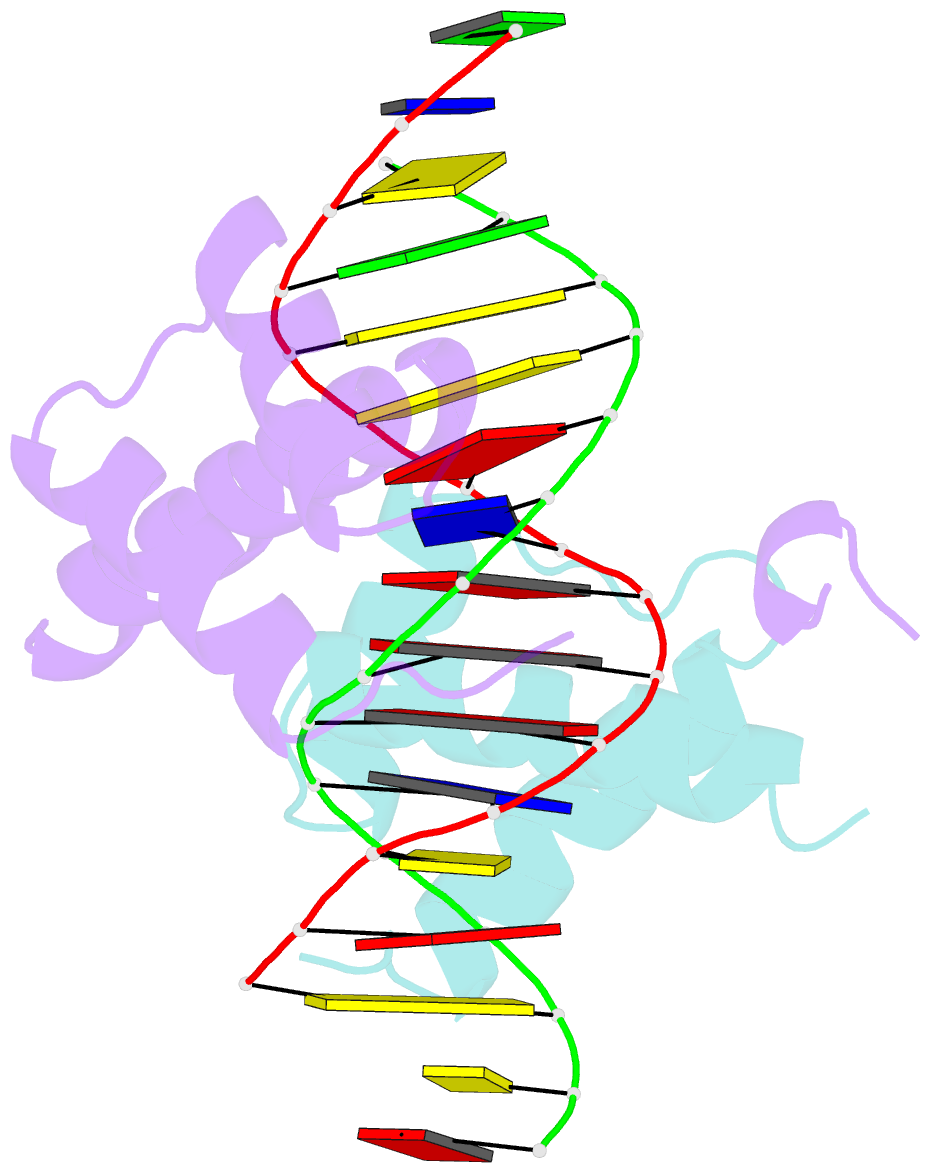
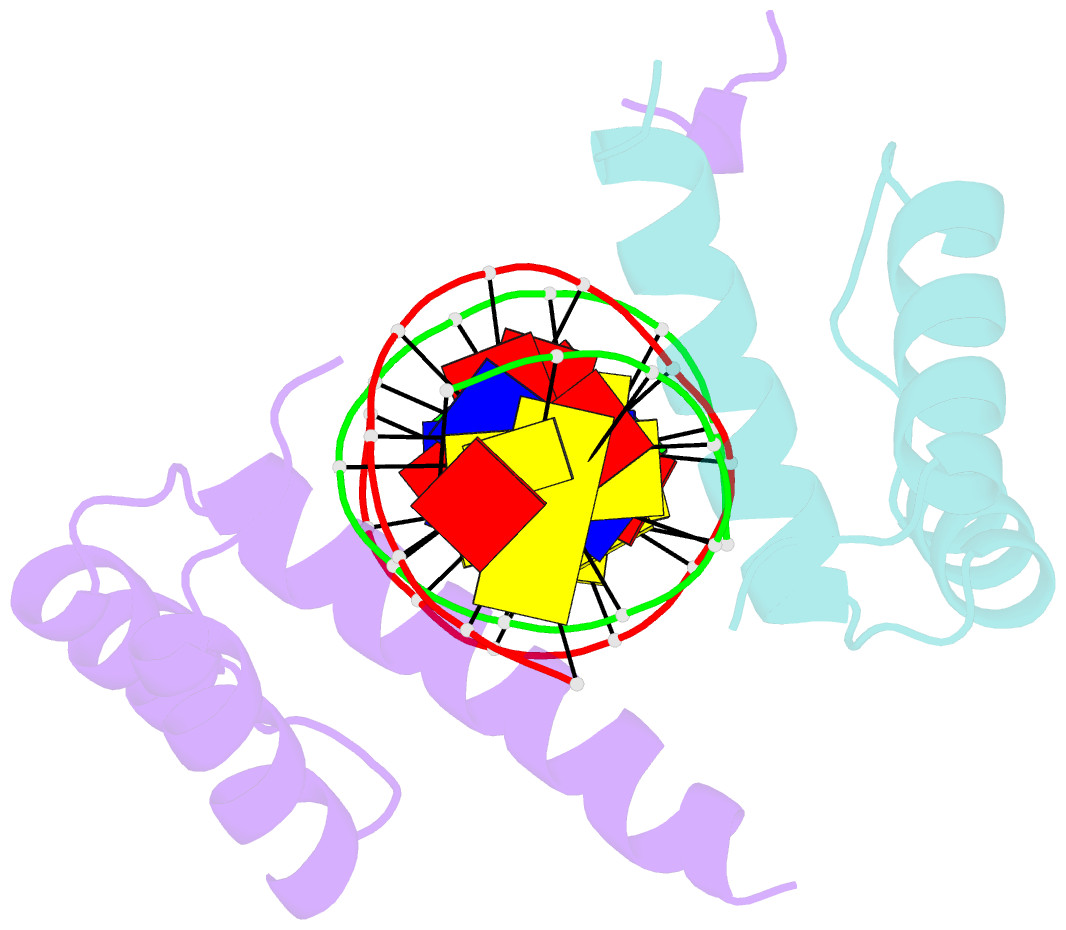
Summary information and primary citation
- PDB-id
- 4cyc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (2.36 Å)
- Summary
- Crystal structure of a ubx-exd-DNA complex including the hexapeptide and ubda motifs
- Reference
- Foos N, Maurel-Zaffran C, Mate MJ, Vincentelli R, Hainaut M, Berenger H, Pradel J, Saurin AJ, Ortiz-Lombardia M, Graba Y (2015): "A Flexible Extension of the Drosophila Ultrabithorax Homeodomain Defines a Novel Hox/Pbc Interaction Mode." Structure, 23, 270. doi: 10.1016/J.STR.2014.12.011.
- Abstract
- The patterning function of Hox proteins relies on assembling protein complexes with PBC proteins, which often involves a protein motif found in most Hox proteins, the so-called Hexapeptide (HX). Hox/PBC complexes likely gained functional diversity by acquiring additional modes of interaction. Here, we structurally characterize the first HX alternative interaction mode based on the paralogue-specific UbdA motif and further functionally validate structure-based predictions. The UbdA motif folds as a flexible extension of the homeodomain recognition helix and defines Hox/PBC contacts that occur, compared with those mediated by the HX motif, on the opposing side of the DNA double helix. This provides a new molecular facet to Hox/PBC complex assembly and suggests possible mechanisms for the diversification of Hox protein function.