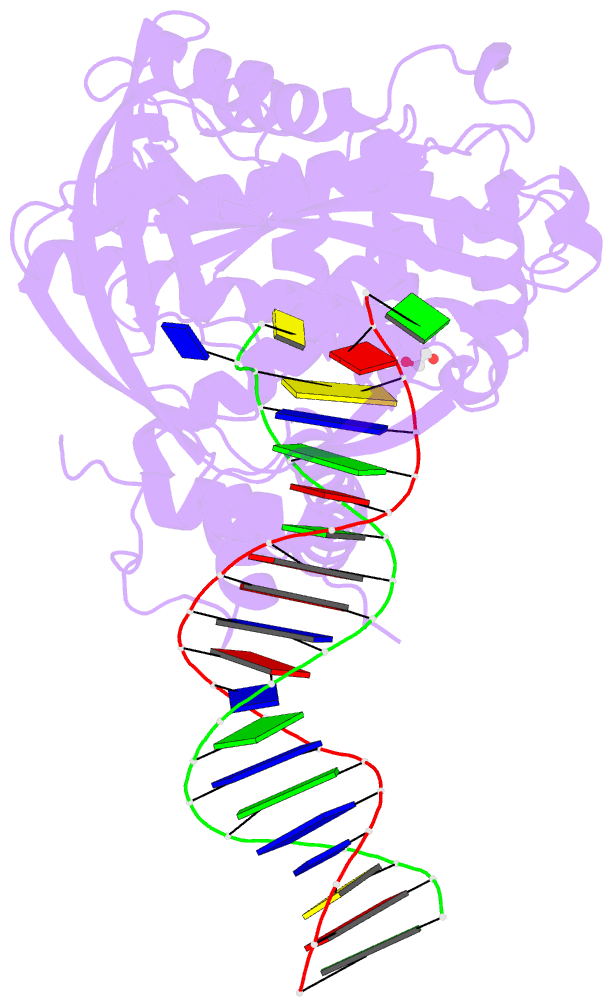
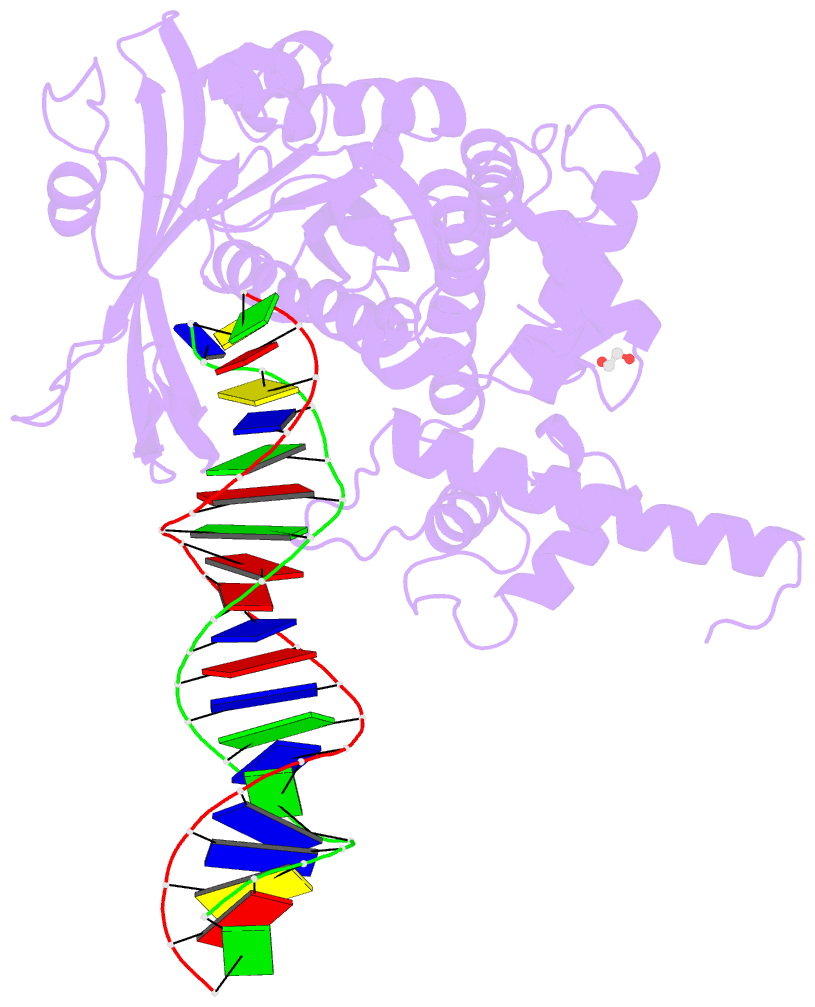
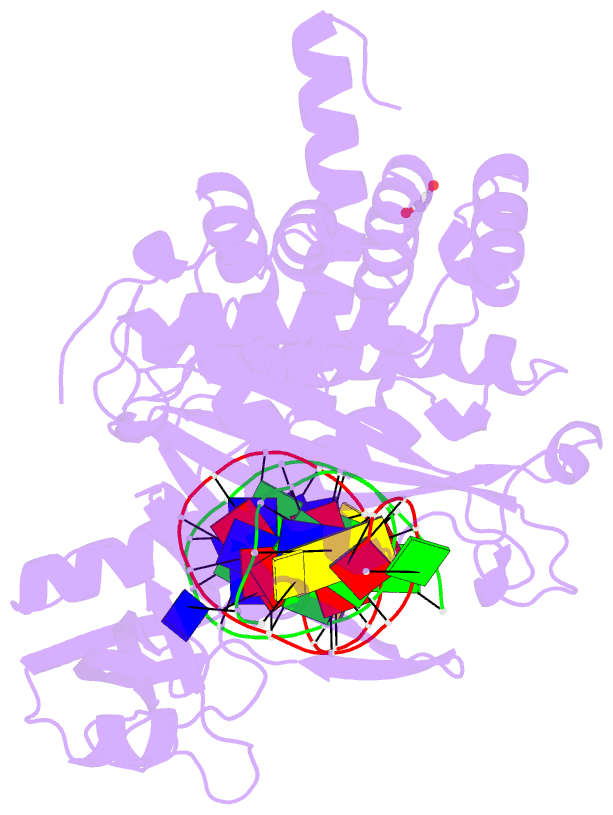
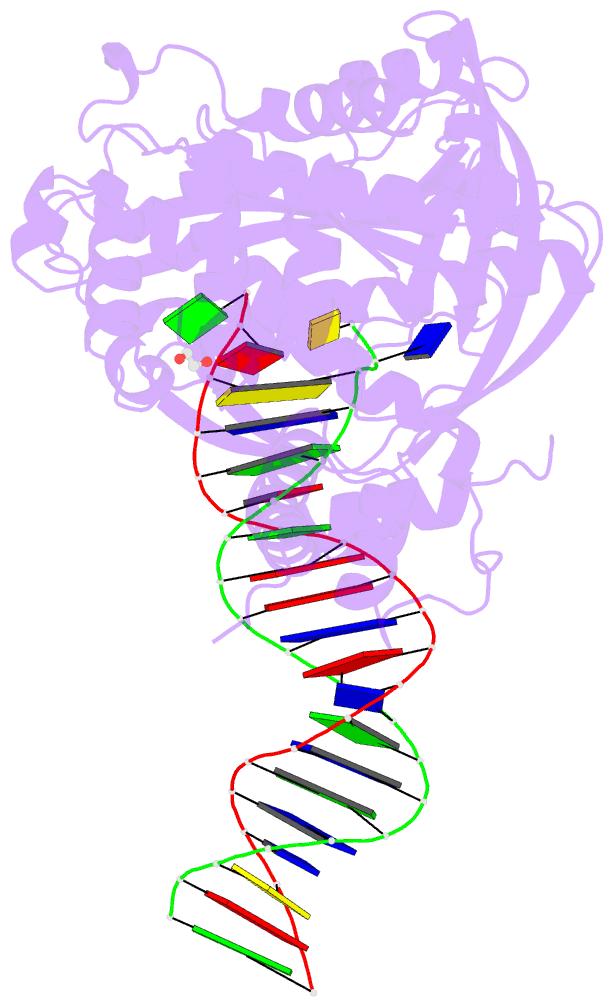
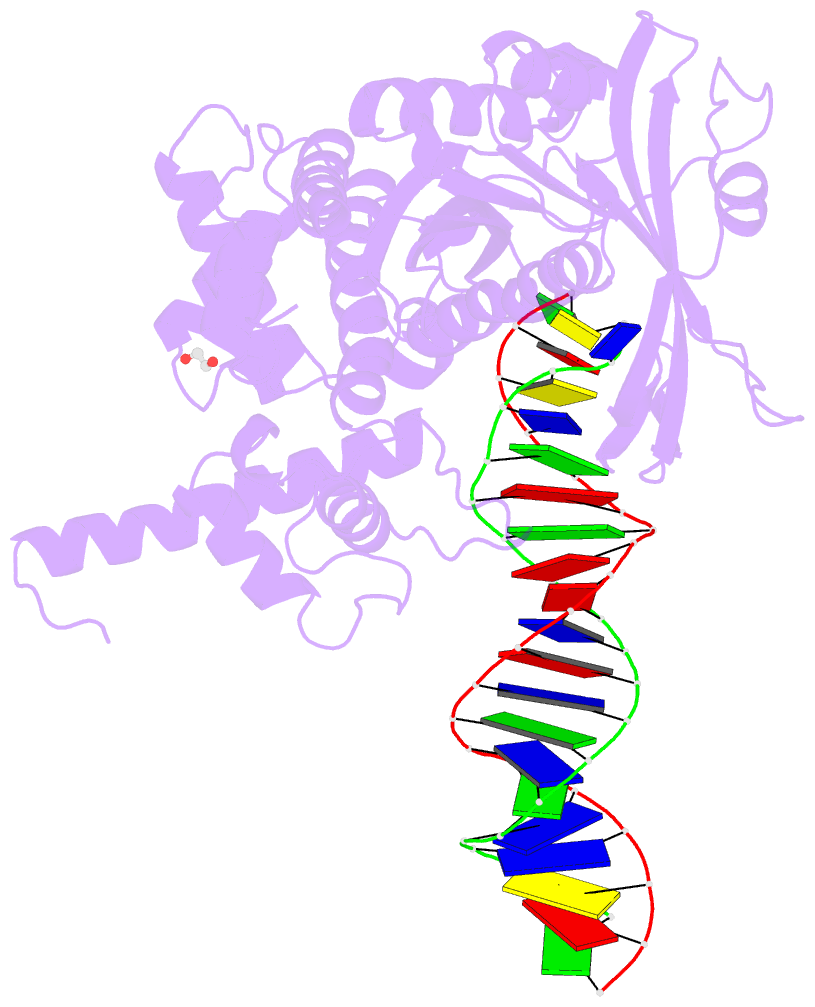
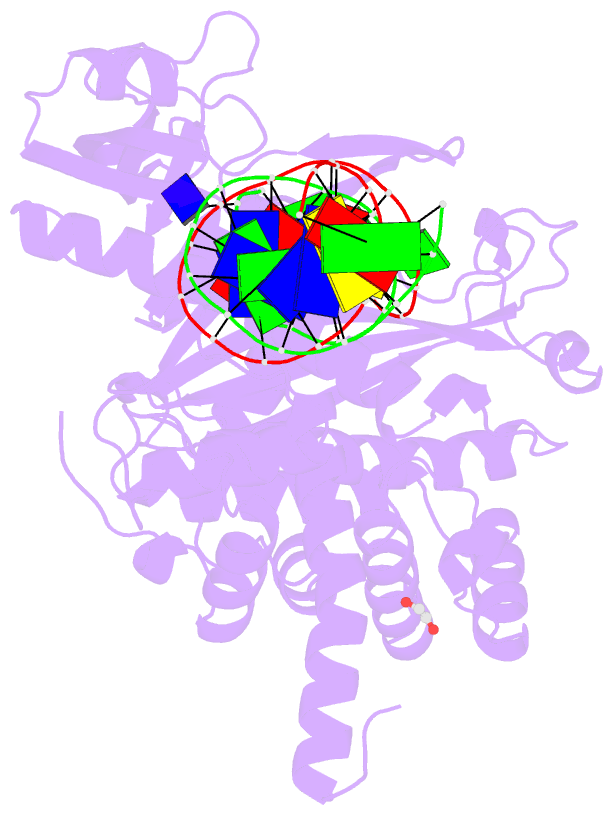
Summary information and primary citation
- PDB-id
- 4dm0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.5 Å)
- Summary
- Tn5 transposase: 20mer outside end 2 mn complex
- Reference
- Lovell S, Goryshin IY, Reznikoff WR, Rayment I (2002): "Two-metal active site binding of a TN5 transposase synaptic complex." Nat.Struct.Biol., 9, 278-281.
- Abstract
- A synaptic complex of Tn5 transposase with an extended outside end DNA duplex was prepared and crystallized, and its crystal structure was determined in an effort to reveal the role of metal ions in catalysis. Two Mn2+ ions bound to the active site when a single nucleotide of donor DNA was added to the 3' end of the transferred strand. Marked conformational changes were observed in the DNA bases closest to the active site. The position of the metal ions and the conformational changes of the DNA provide insight into the mechanism of hairpin formation and cleavage, and is consistent with a two-metal model for catalysis.