Summary information and primary citation
- PDB-id
- 4e68; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.585 Å)
- Summary
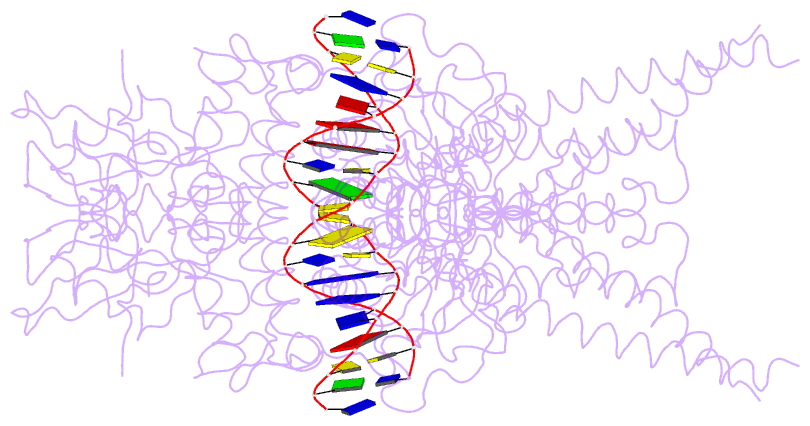
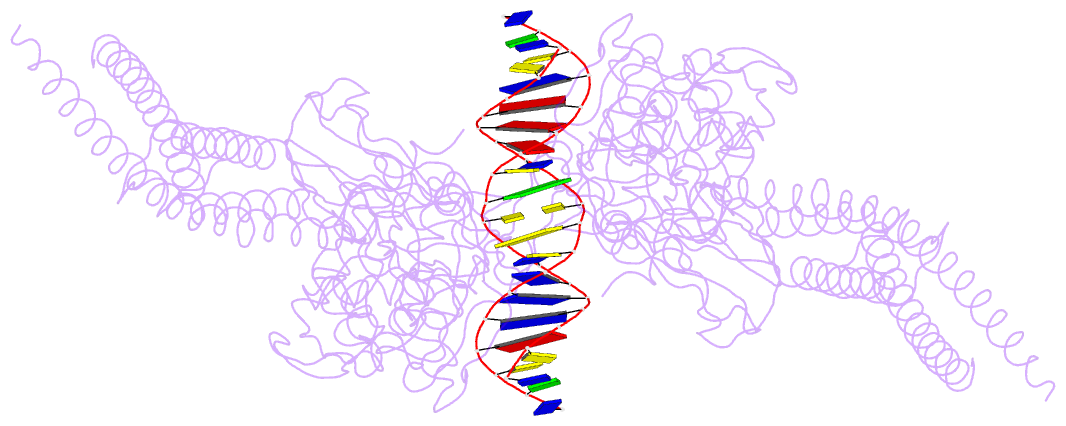
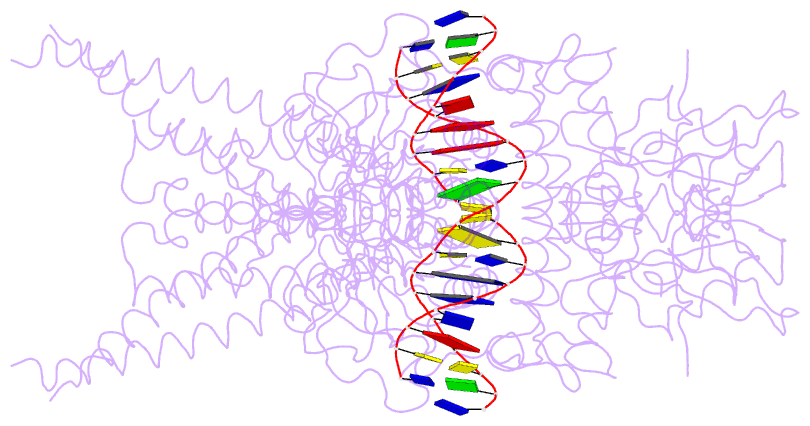
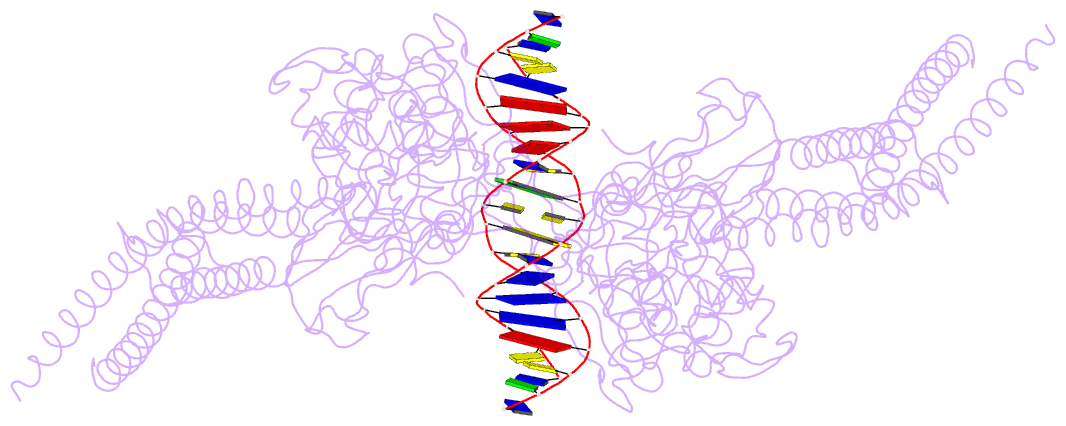
- Unphosphorylated stat3b core protein binding to dsDNA
- Reference
- Nkansah E, Shah R, Collie GW, Parkinson GN, Palmer J, Rahman KM, Bui TT, Drake AF, Husby J, Neidle S, Zinzalla G, Thurston DE, Wilderspin AF (2013): "Observation of unphosphorylated STAT3 core protein binding to target dsDNA by PEMSA and X-ray crystallography." Febs Lett., 587, 833-839. doi: 10.1016/j.febslet.2013.01.065.
- Abstract
- The STAT3 transcription factor plays a central role in a wide range of cancer types where it is over-expressed. Previously, phosphorylation of this protein was thought to be a prerequisite for direct binding to DNA. However, we have now shown complete binding of a purified unphosphorylated STAT3 (uSTAT3) core directly to M67 DNA, the high affinity STAT3 target DNA sequence, by a protein electrophoretic mobility shift assay (PEMSA). Binding to M67 DNA was inhibited by addition of increasing concentrations of a phosphotyrosyl peptide. X-ray crystallography demonstrates one mode of binding that is similar to that known for the STAT3 core phosphorylated at Y705.