Summary information and primary citation
- PDB-id
- 4ed5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.0 Å)
- Summary
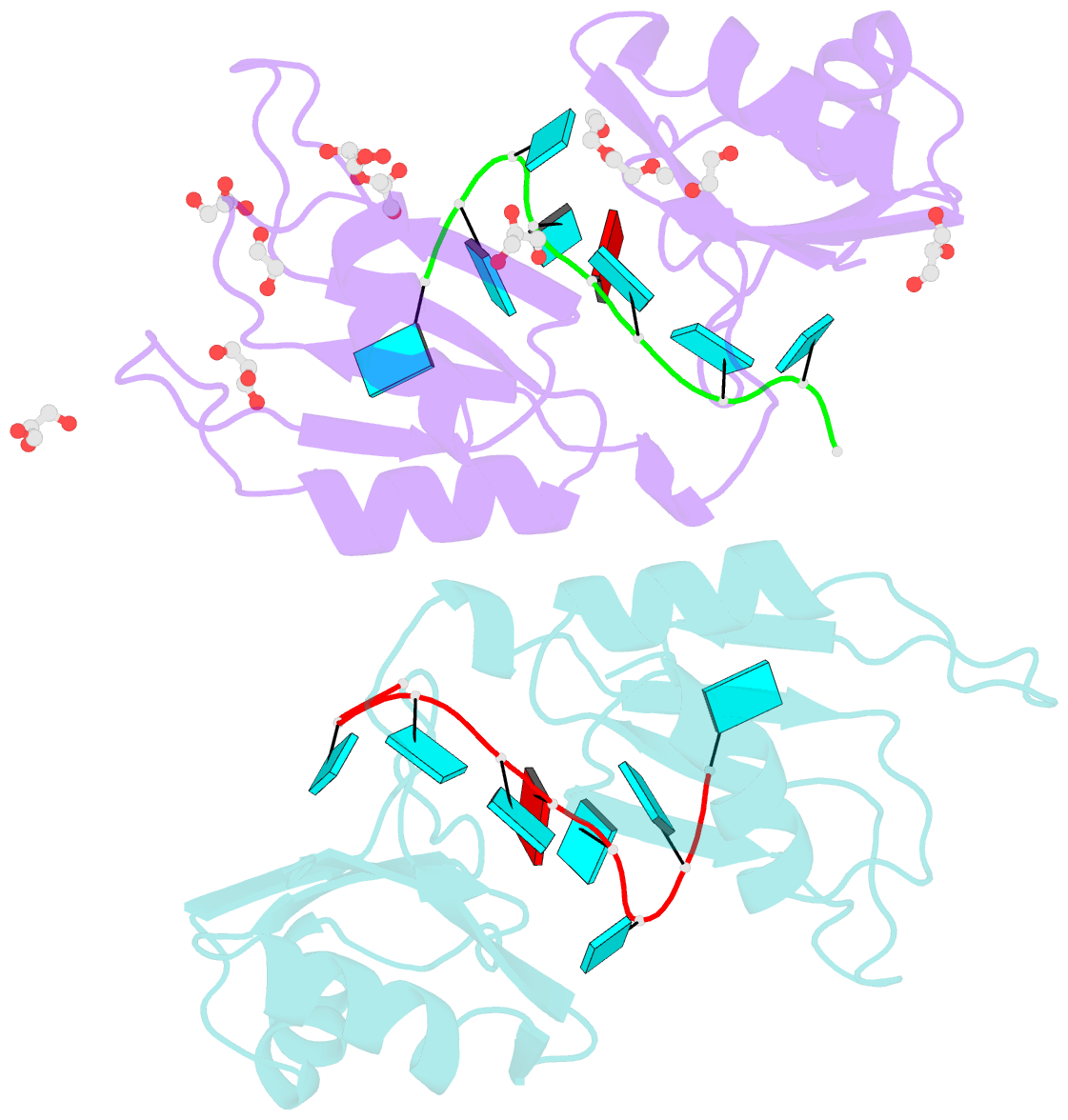
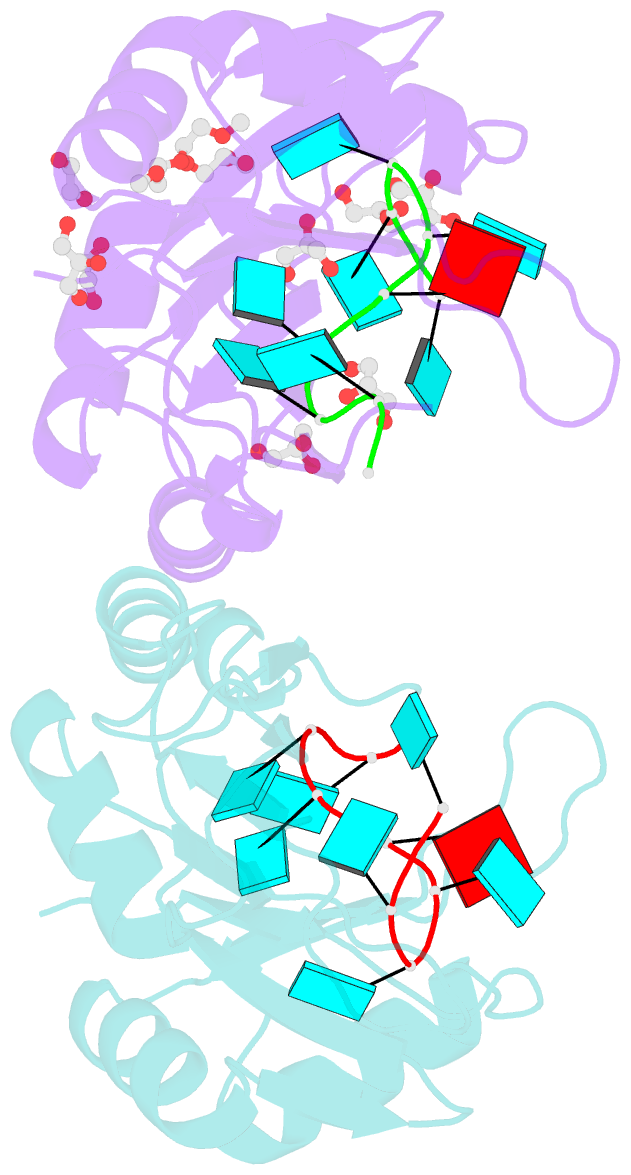
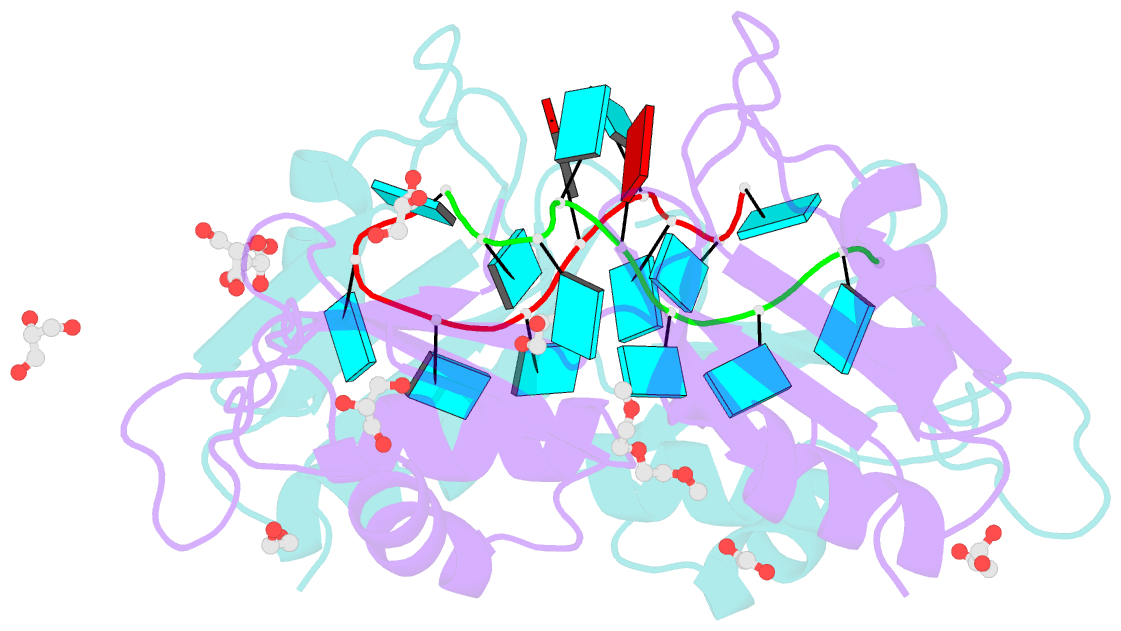
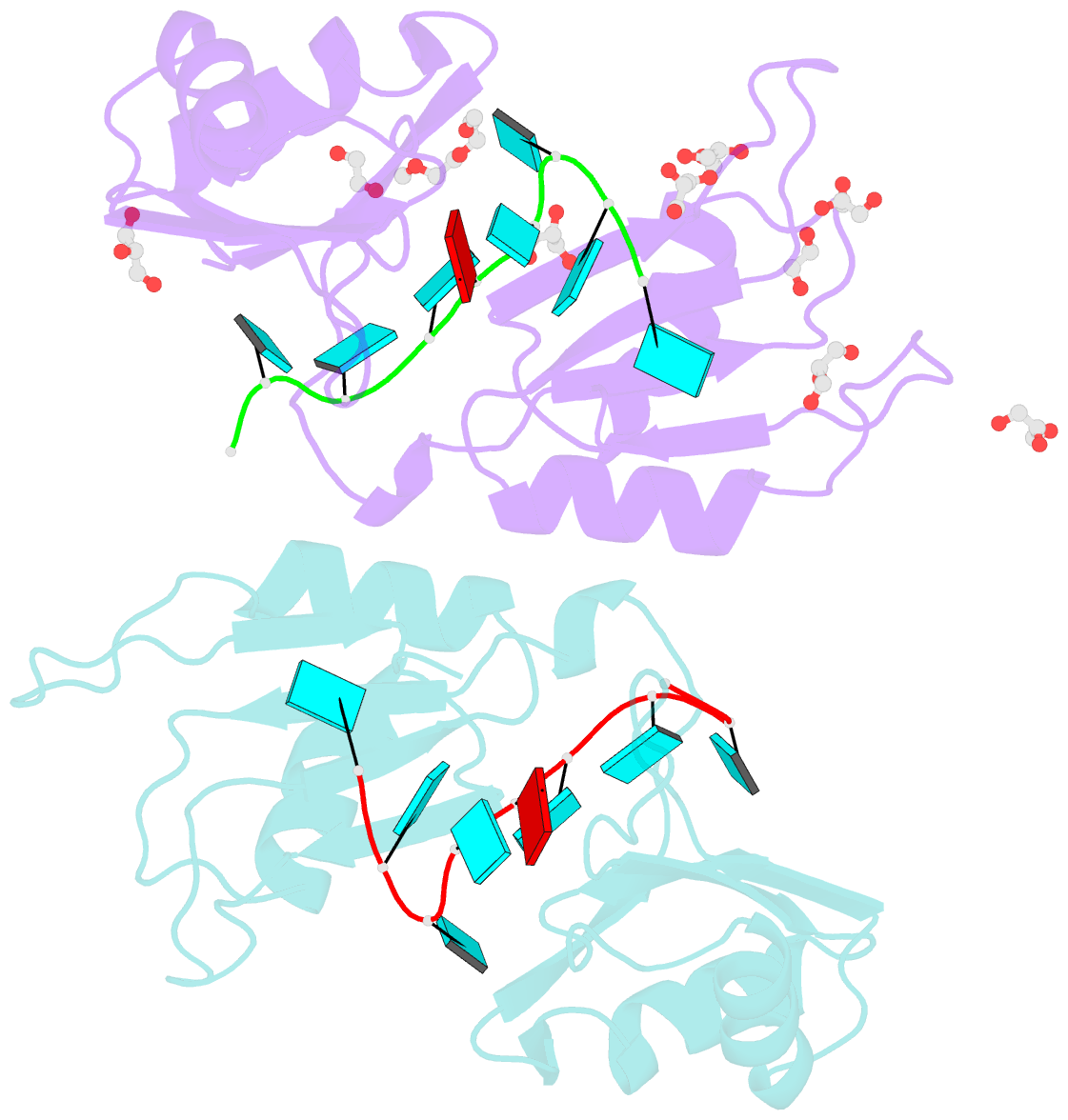
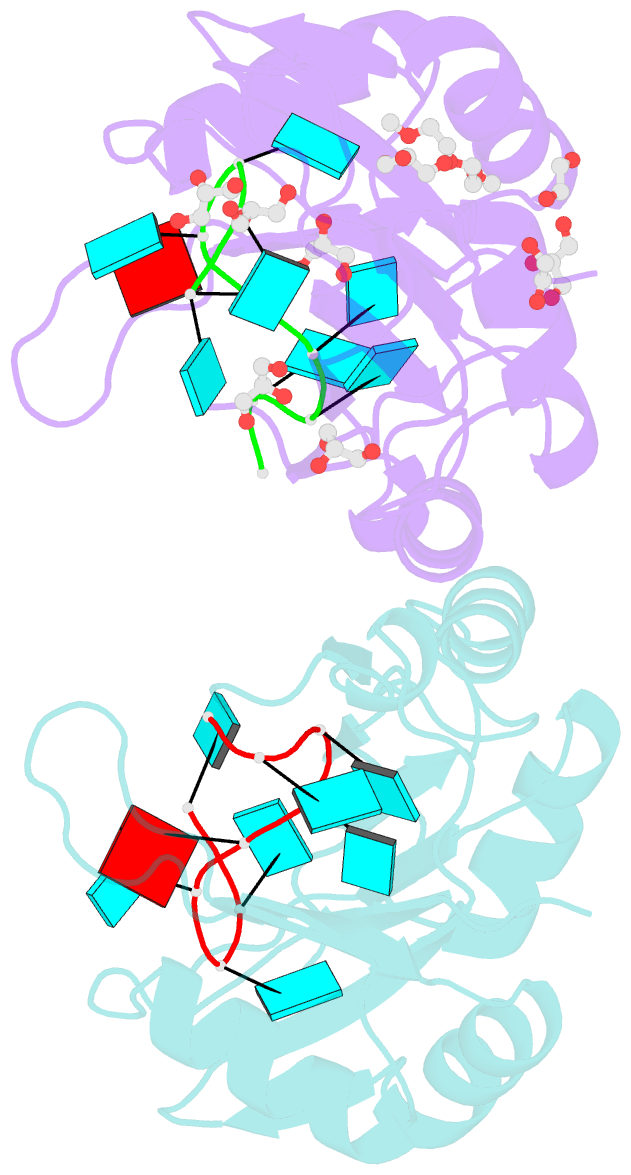
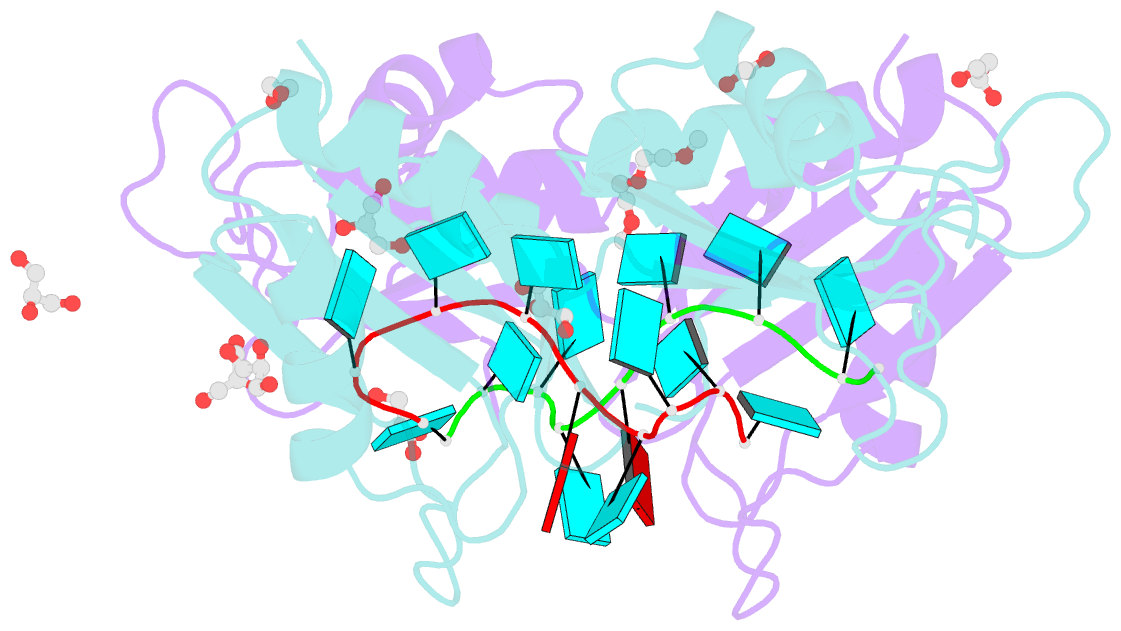
- Crystal structure of the two n-terminal rrm domains of hur complexed with RNA
- Reference
- Wang H, Zeng F, Liu Q, Liu H, Liu Z, Niu L, Teng M, Li X (2013): "The structure of the ARE-binding domains of Hu antigen R (HuR) undergoes conformational changes during RNA binding." Acta Crystallogr.,Sect.D, 69, 373-380. doi: 10.1107/S0907444912047828.
- Abstract
- Human RNA-binding protein (HuR), a ubiquitously expressed member of the Hu protein family, plays an important role in mRNA degradation and has been implicated as a key post-transcriptional regulator. HuR contains three RNA-recognition motif (RRM) domains. The two N-terminal tandem RRM domains can selectively bind AU-rich elements (AREs), while the third RRM domain (RRM3) contributes to interactions with the poly-A tail of target mRNA and other ligands. Here, the X-ray structure of two methylated tandem RRM domains (RRM1/2) of HuR in their RNA-free form was solved at 2.9 Å resolution. The crystal structure of RRM1/2 complexed with target mRNA was also solved at 2.0 Å resolution; comparisons of the two structures show that HuR RRM1/2 undergoes conformational changes upon RNA binding. Fluorescence polarization assays (FPA) were used to study the protein-RNA interactions. Both the structure and the FPA analysis indicated that RRM1 is the primary ARE-binding domain in HuR and that the conformational changes induce subsequent contacts of the RNA substrate with the inter-domain linker and RRM2 which greatly improve the RNA-binding affinity of HuR.