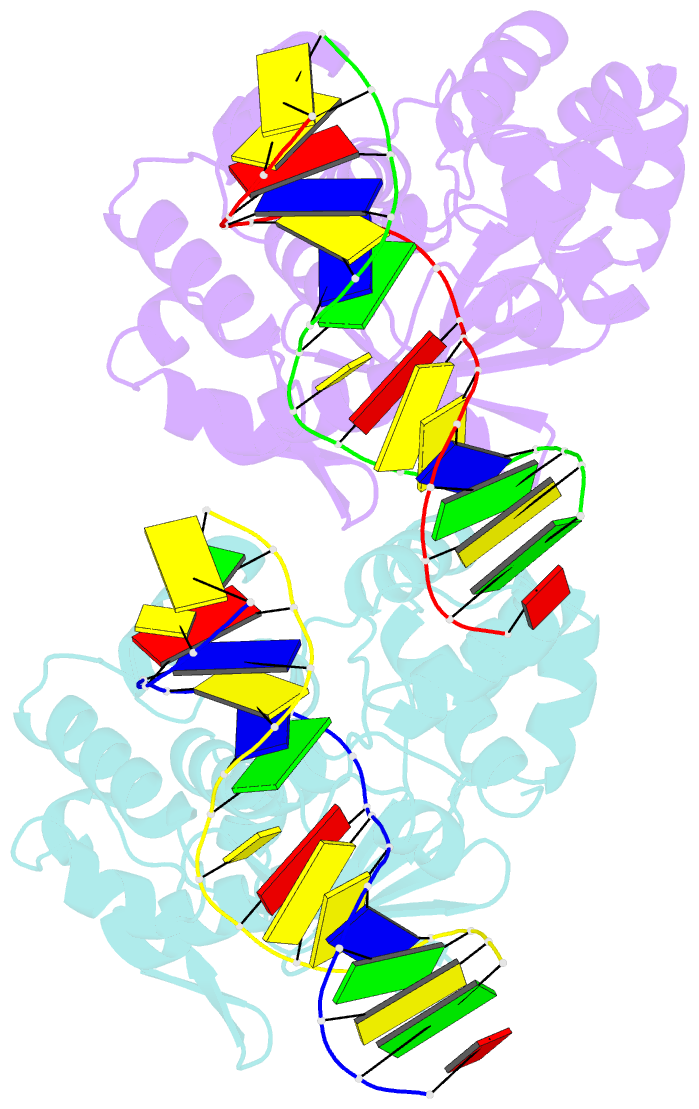
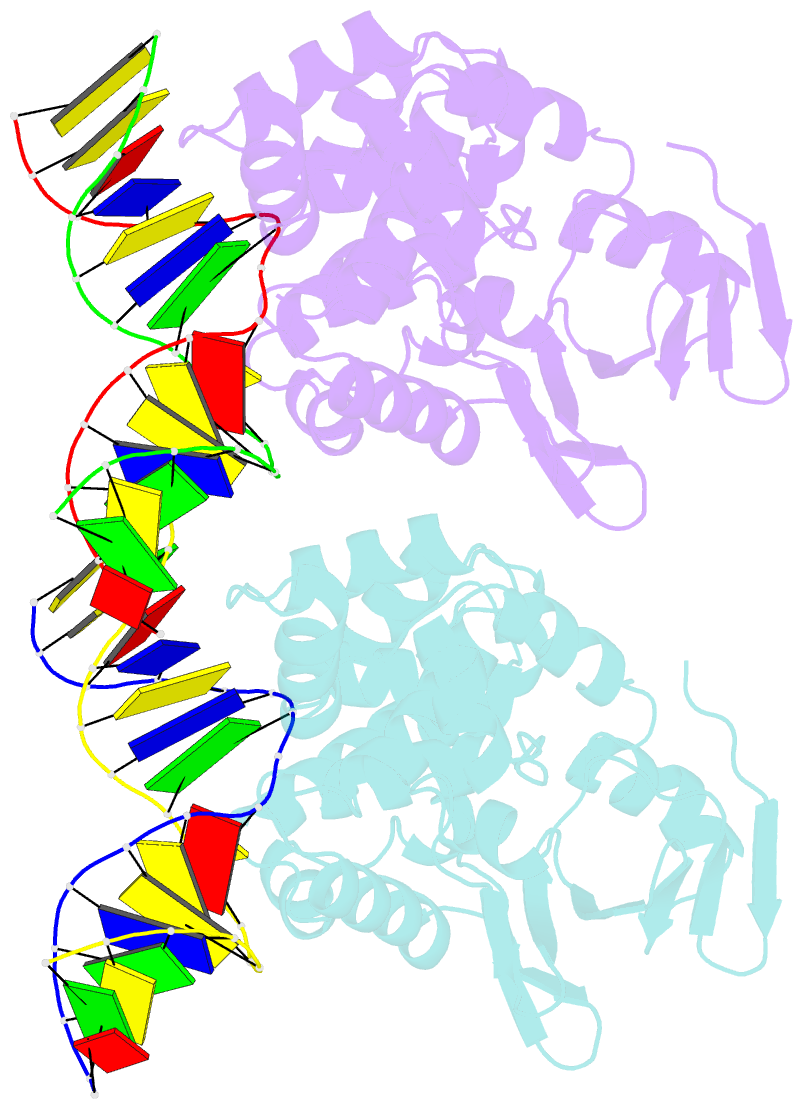
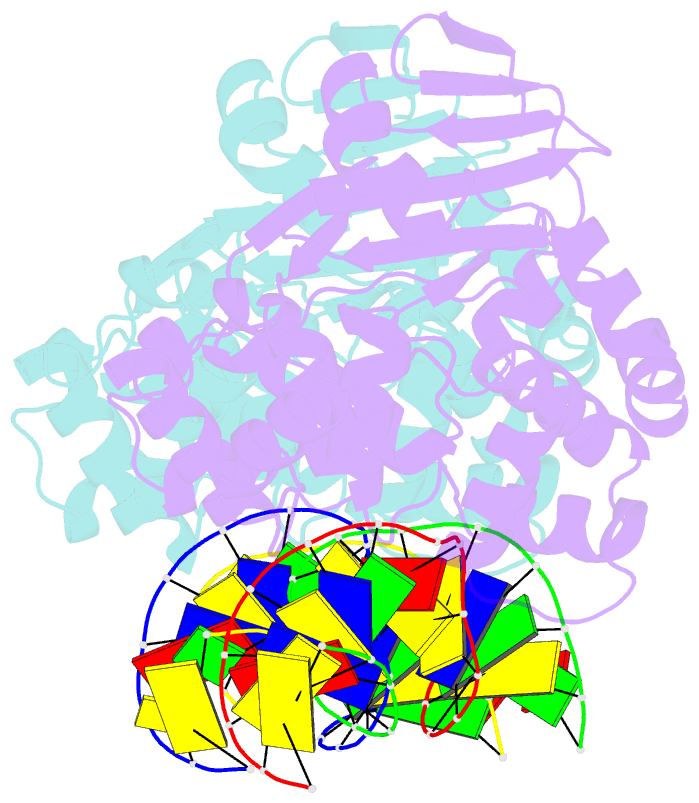
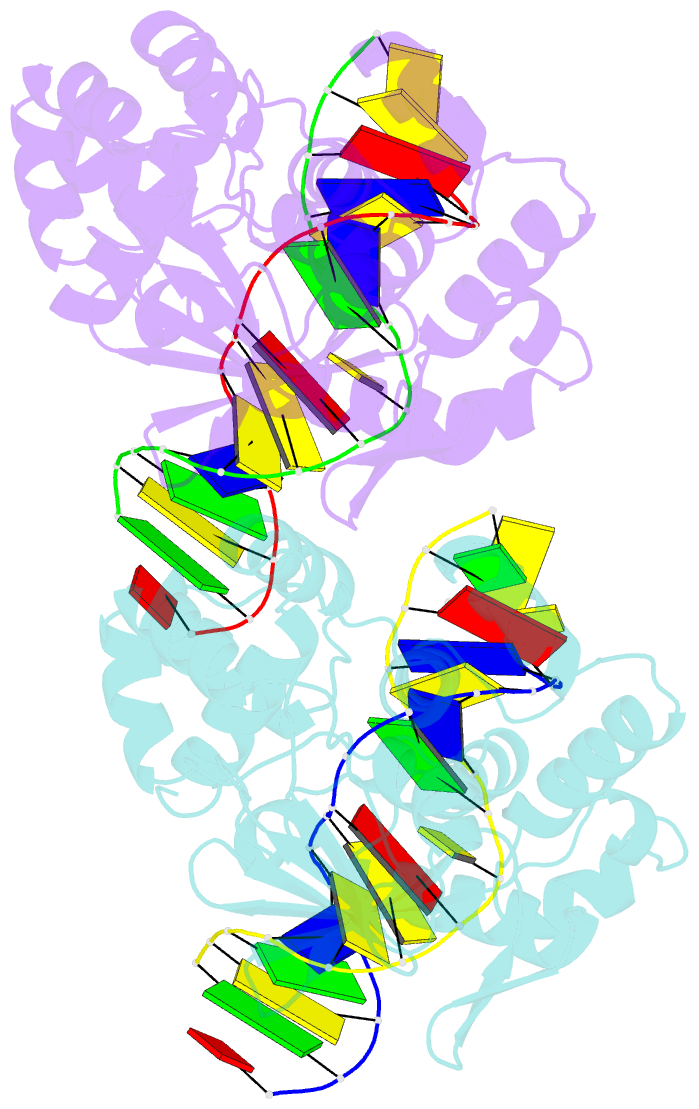
Summary information and primary citation
- PDB-id
- 4ejy; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.0 Å)
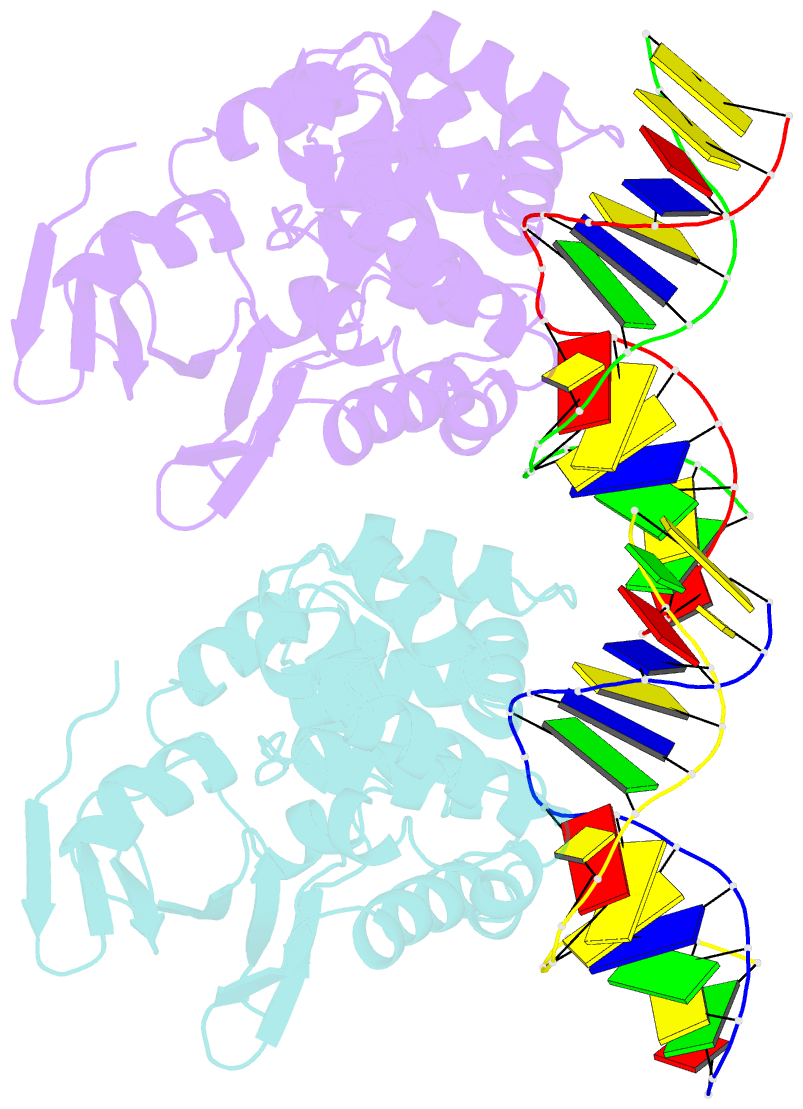
- Summary
- Structure of mbogg1 in complex with high affinity DNA ligand
- Reference
- Yu HJ, Yang MZ, Zhang XE, Bi LJ, Jiang T (2013): "Crystal structures of MBOgg1 in complex with two abasic DNA ligands." J.Struct.Biol., 181, 252-263. doi: 10.1016/j.jsb.2012.12.003.
- Abstract
- 7,8-Dihydro-8-oxoguanine (8-oxoG) is one of the most common oxidative DNA lesions. 8-oxoguanine DNA glycosylases (Oggs) detect and excise 8-oxoG through a multiple-step process. To better understand the basis for estranged base recognition, we have solved the crystal structures of MBOgg1, the 8-oxoguanine DNA glycosylase of Thermoanaerobacter tengcongensis, in complex with DNA containing a tetrahydrofuranyl site (THF, a stable abasic site analog) paired with an estranged cytosine (MBOgg1/DNA(THF:C)) or thymine (MBOgg1/DNA(THF:T)). Different states of THF (extrahelical or intrahelical) are observed in the two complexes of the ASU of MBOgg1/DNA(THF:C) structure. Analyses of their different interaction modes reveal that variable contacts on the 5' region flanking the THF abasic site are correlated with the states of the THF. Comparison of MBOgg1/DNA(THF:T) with MBOgg1/DNA(THF:C) indicates that the non-preferred estranged T may affect MBOgg1's contacts with the 5' flank of the lesion strand. Furthermore, we identified a region in MBOgg1 that is rich in positive charges and interacts with the 5' region flanking the lesion. This region is conserved only in non-eukaryotic Oggs, and additional mutagenesis and biochemical assays reveal that it may contribute to the distinct estranged base specificities between eukaryotic and non-eukaryotic Oggs.