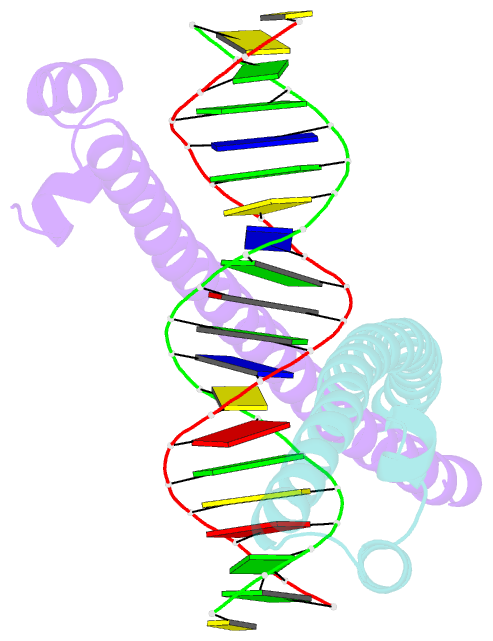
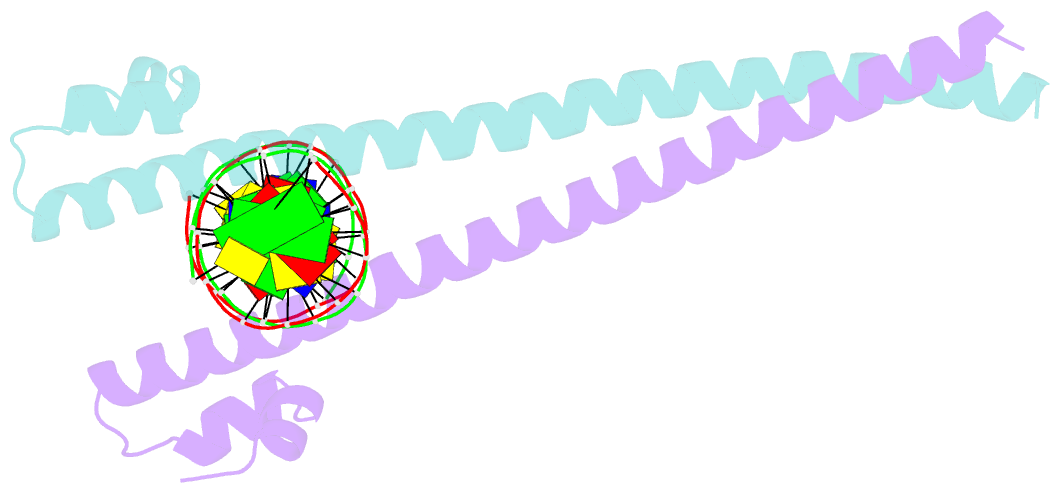
Summary information and primary citation
- PDB-id
- 4eot; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.855 Å)
- Summary
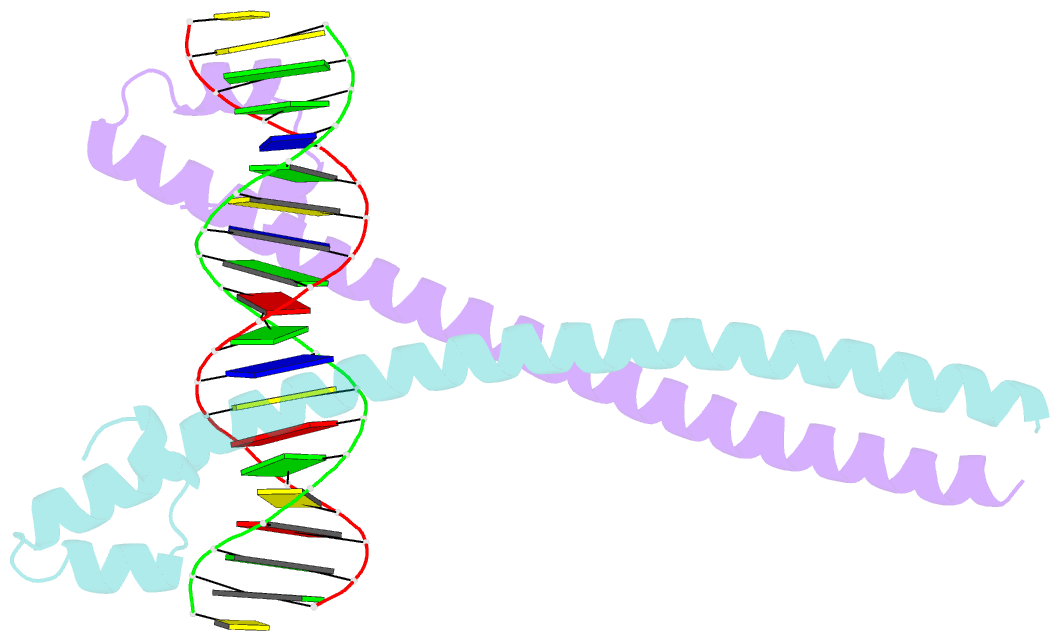
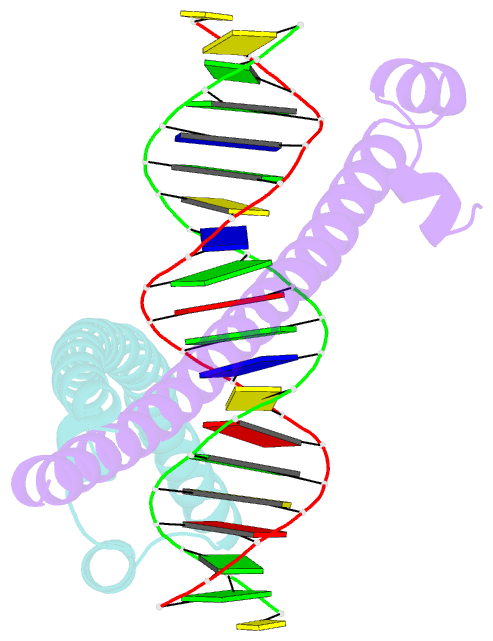
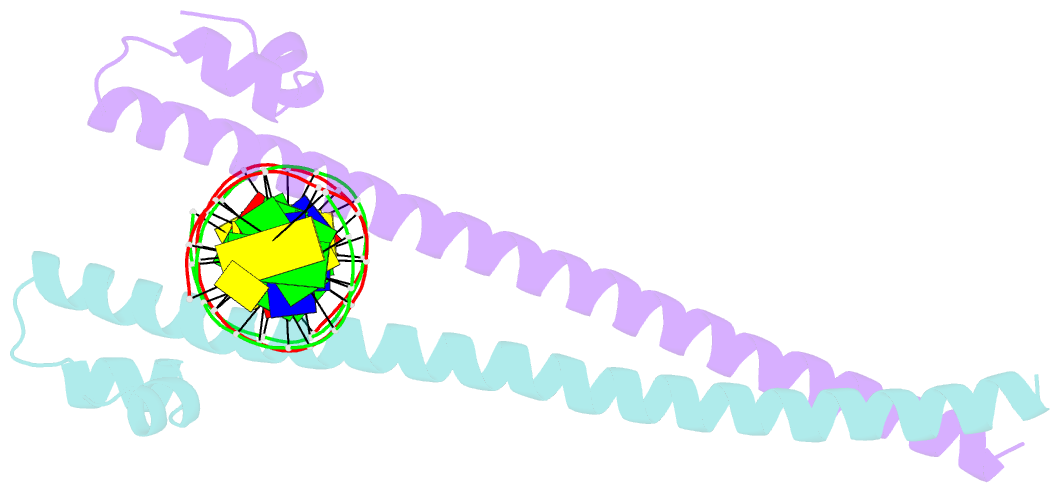
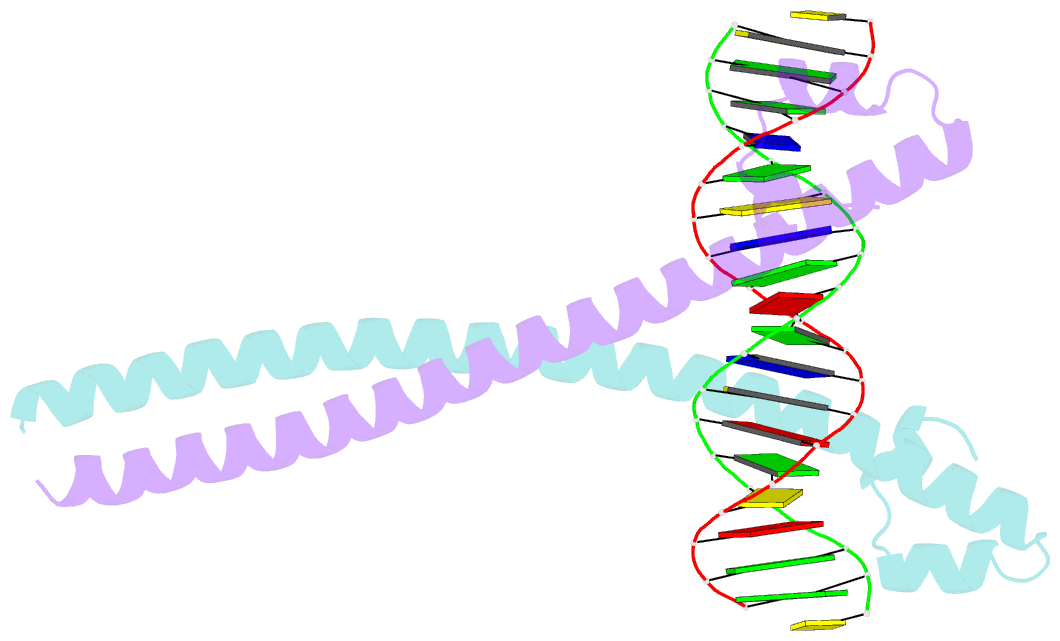
- Crystal structure of the mafa homodimer bound to the consensus mare
- Reference
- Lu X, Guanga GP, Wan C, Rose RB (2012): "A Novel DNA Binding Mechanism for maf Basic Region-Leucine Zipper Factors Inferred from a MafA-DNA Complex Structure and Binding Specificities." Biochemistry, 51, 9706-9717. doi: 10.1021/bi301248j.
- Abstract
- MafA is a proto-oncoprotein and is critical for insulin gene expression in pancreatic β-cells. Maf proteins belong to the AP1 superfamily of basic region-leucine zipper (bZIP) transcription factors. Residues in the basic helix and an ancillary N-terminal domain, the Extended Homology Region (EHR), endow maf proteins with unique DNA binding properties: binding a 13 bp consensus site consisting of a core AP1 site (TGACTCA) flanked by TGC sequences and binding DNA stably as monomers. To further characterize maf DNA binding, we determined the structure of a MafA-DNA complex. MafA forms base-specific hydrogen bonds with the flanking G(-5)C(-4) and central C(0)/G(0) bases, but not with the core-TGA bases. However, in vitro binding studies utilizing a pulse-chase electrophoretic mobility shift assay protocol revealed that mutating either the core-TGA or flanking-TGC bases dramatically increases the binding off rate. Comparing the known maf structures, we propose that DNA binding specificity results from positioning the basic helix through unique phosphate contacts. The EHR does not contact DNA directly but stabilizes DNA binding by contacting the basic helix. Collectively, these results suggest a novel multistep DNA binding process involving a conformational change from contacting the core-TGA to contacting the flanking-TGC bases.