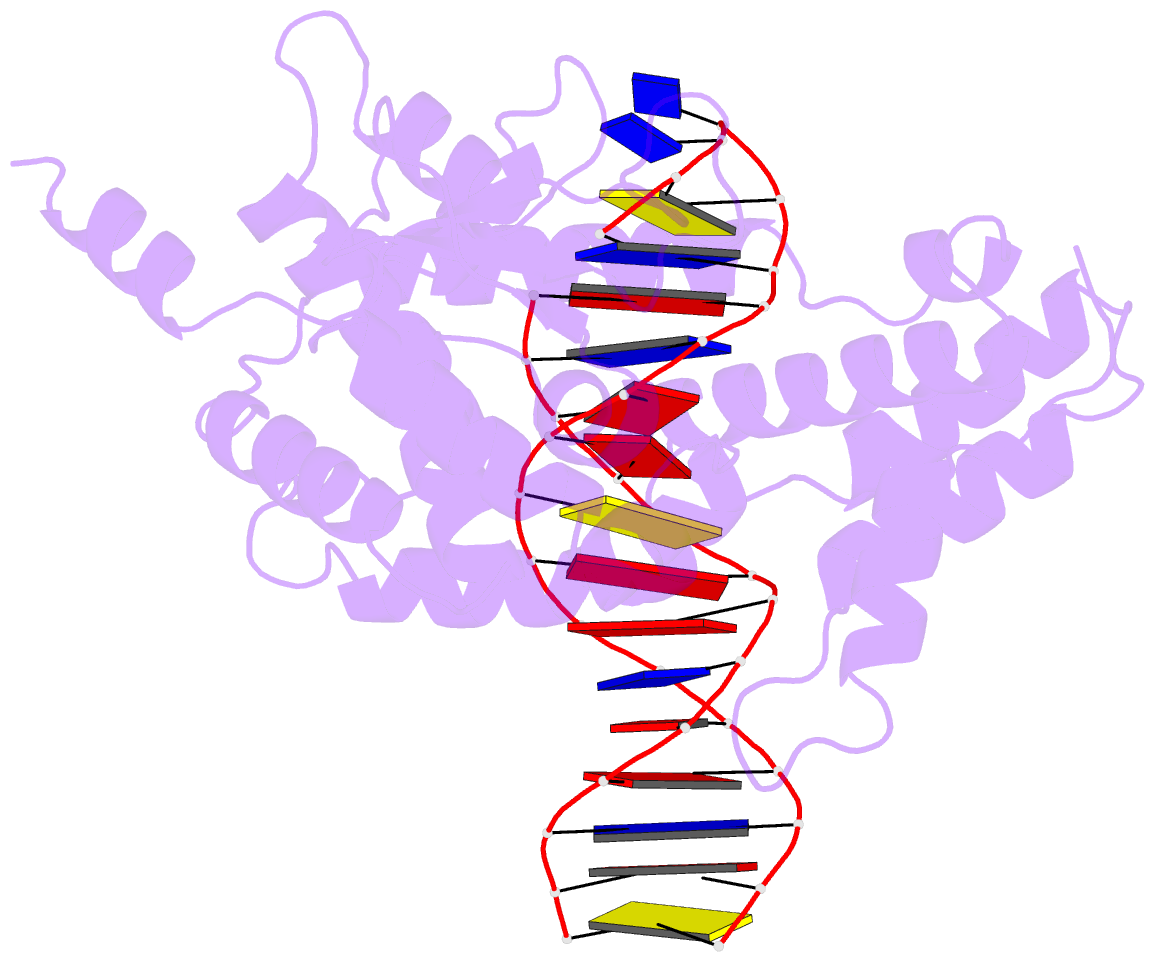
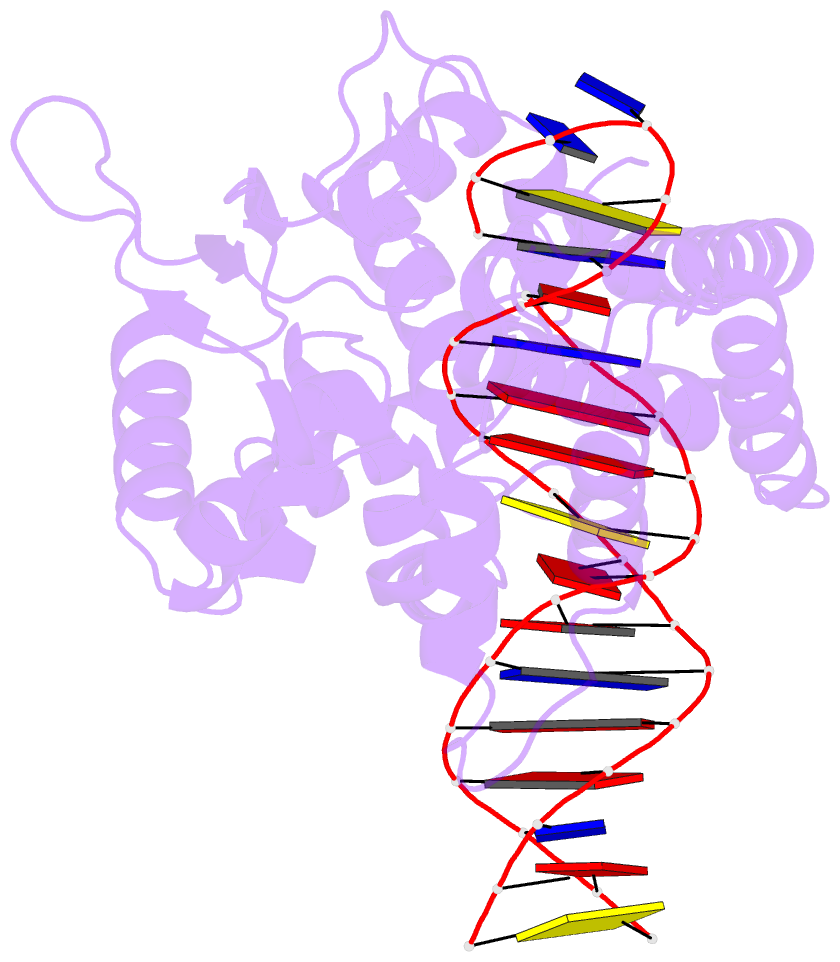
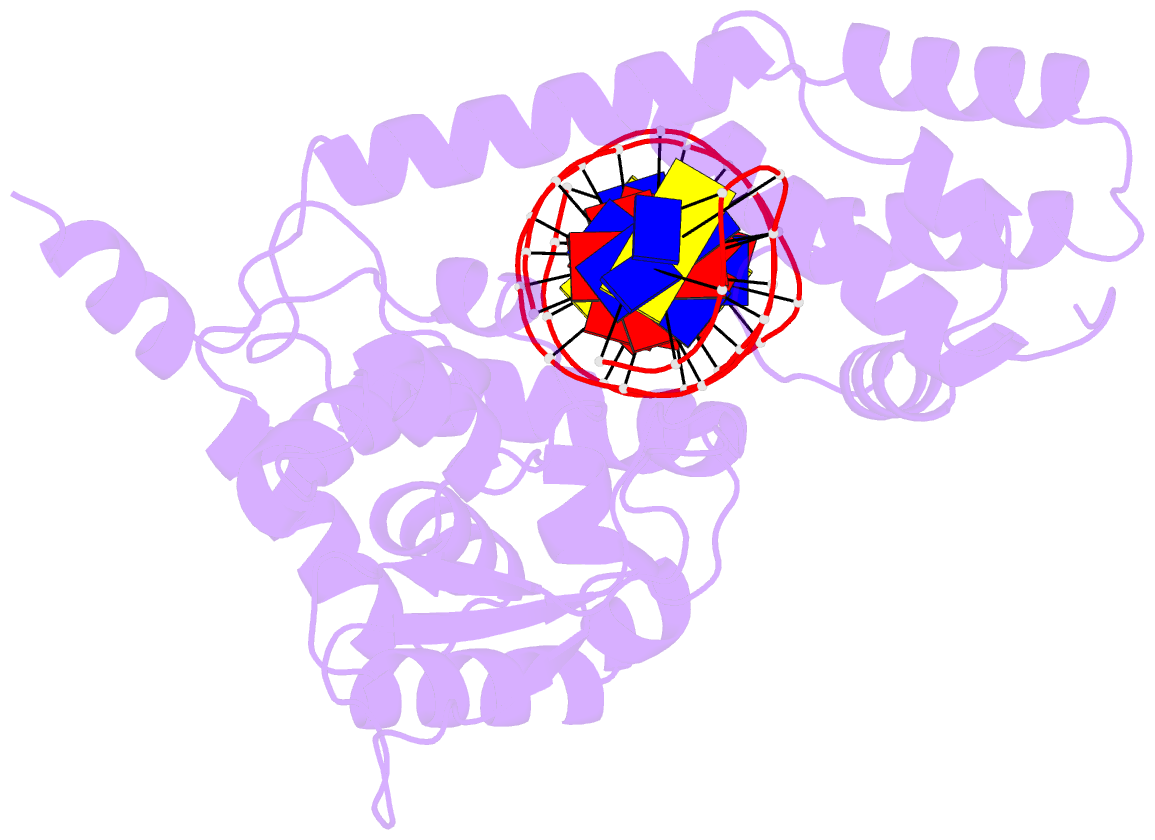
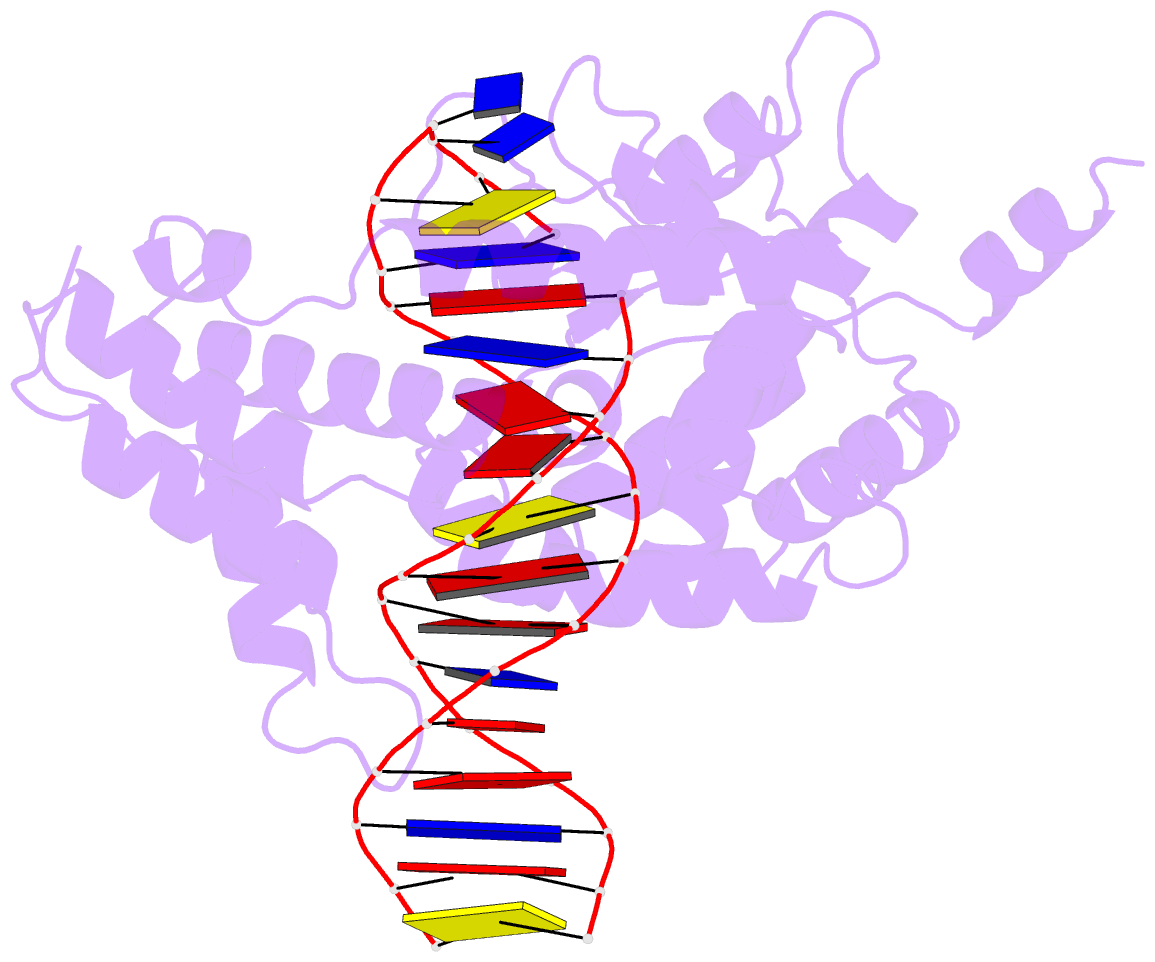
Summary information and primary citation
- PDB-id
- 4f41; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- recombination-DNA
- Method
- X-ray (2.5 Å)
- Summary
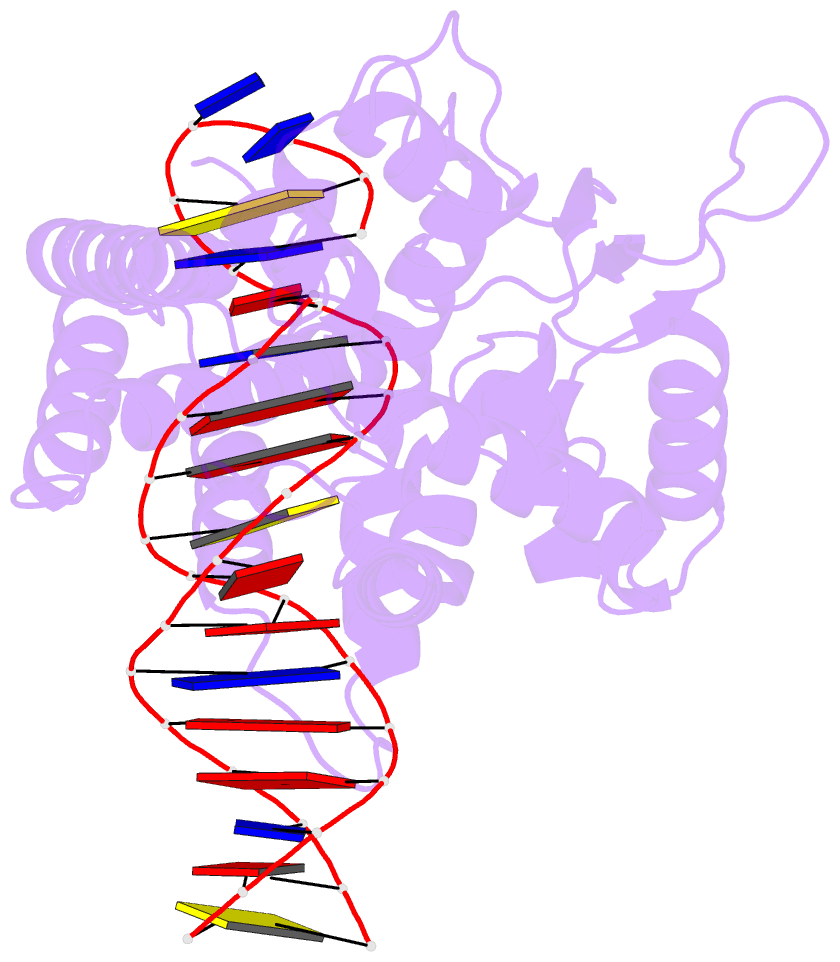
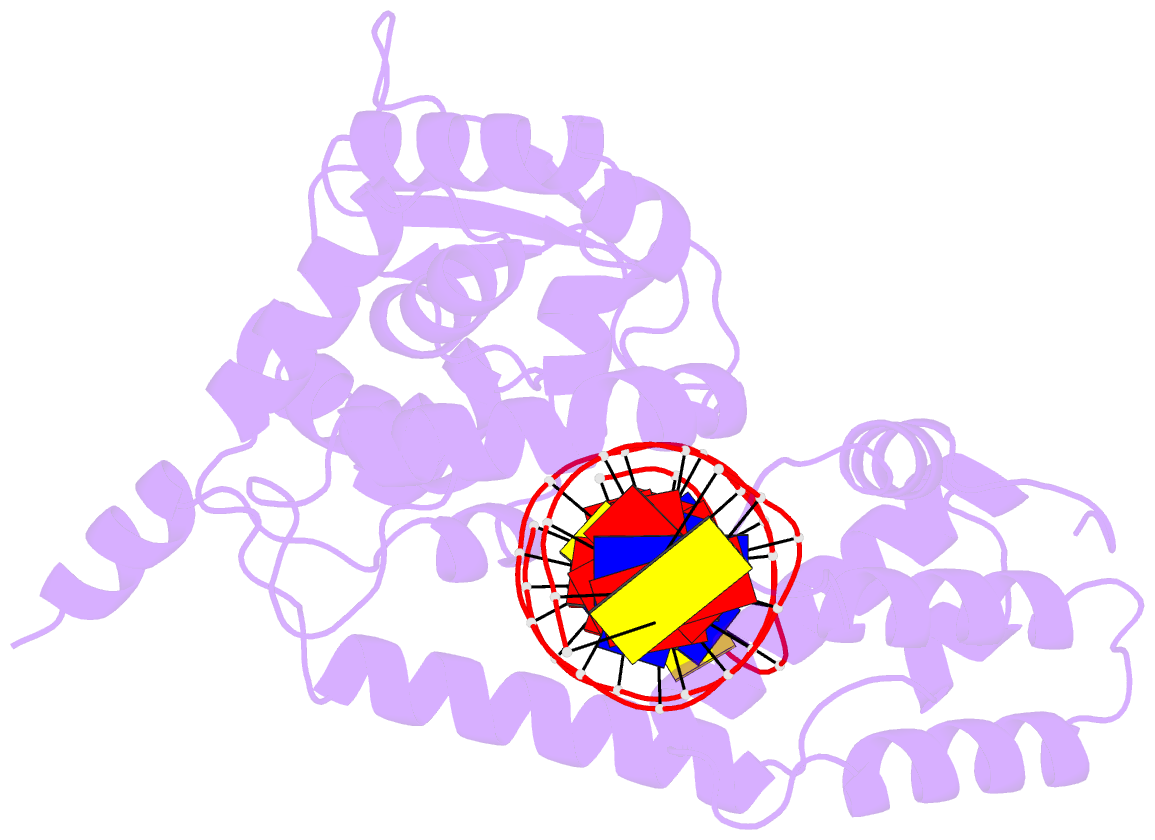
- Protelomerase tela mutant r255a complexed with cttg hairpin DNA
- Reference
- Huang WM, Dagloria J, Fox H, Ruan Q, Tillou J, Shi K, Aihara H, Aron J, Casjens S (2012): "Linear Chromosome-generating System of Agrobacterium tumefaciens C58: PROTELOMERASE GENERATES AND PROTECTS HAIRPIN ENDS." J.Biol.Chem., 287, 25551-25563. doi: 10.1074/jbc.M112.369488.
- Abstract
- Agrobacterium tumefaciens C58, the pathogenic bacteria that causes crown gall disease in plants, harbors one circular and one linear chromosome and two circular plasmids. The telomeres of its unusual linear chromosome are covalently closed hairpins. The circular and linear chromosomes co-segregate and are stably maintained in the organism. We have determined the sequence of the two ends of the linear chromosome thus completing the previously published genome sequence of A. tumefaciens C58. We found that the telomeres carry nearly identical 25-bp sequences at the hairpin ends that are related by dyad symmetry. We further showed that its Atu2523 gene encodes a protelomerase (resolvase) and that the purified enzyme can generate the linear chromosomal closed hairpin ends in a sequence-specific manner. Agrobacterium protelomerase, whose presence is apparently limited to biovar 1 strains, acts via a cleavage-and-religation mechanism by making a pair of transient staggered nicks invariably at 6-bp spacing as the reaction intermediate. The enzyme can be significantly shortened at both the N and C termini and still maintain its enzymatic activity. Although the full-length enzyme can uniquely bind to its product telomeres, the N-terminal truncations cannot. The target site can also be shortened from the native 50-bp inverted repeat to 26 bp; thus, the Agrobacterium hairpin-generating system represents the most compact activity of all hairpin linear chromosome- and plasmid-generating systems to date. The biochemical analyses of the protelomerase reactions further revealed that the tip of the hairpin telomere may be unusually polymorphically capable of accommodating any nucleotide.