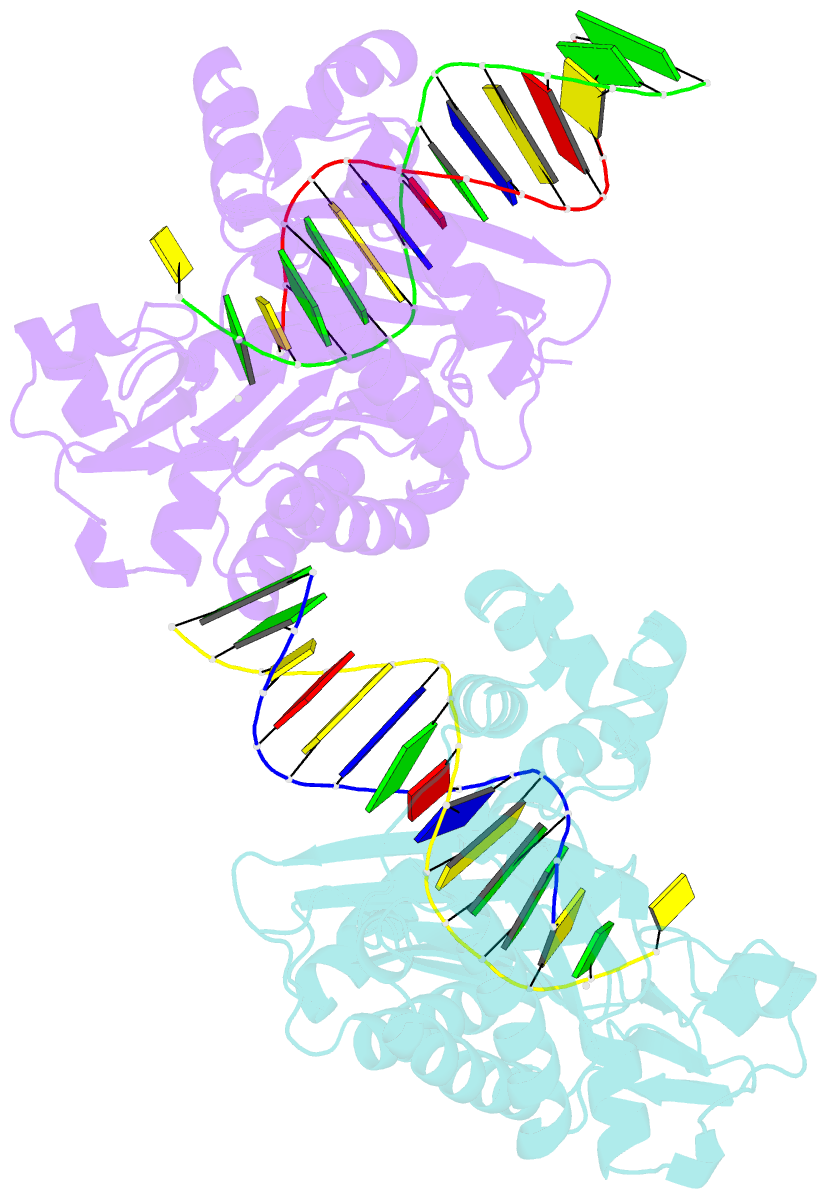
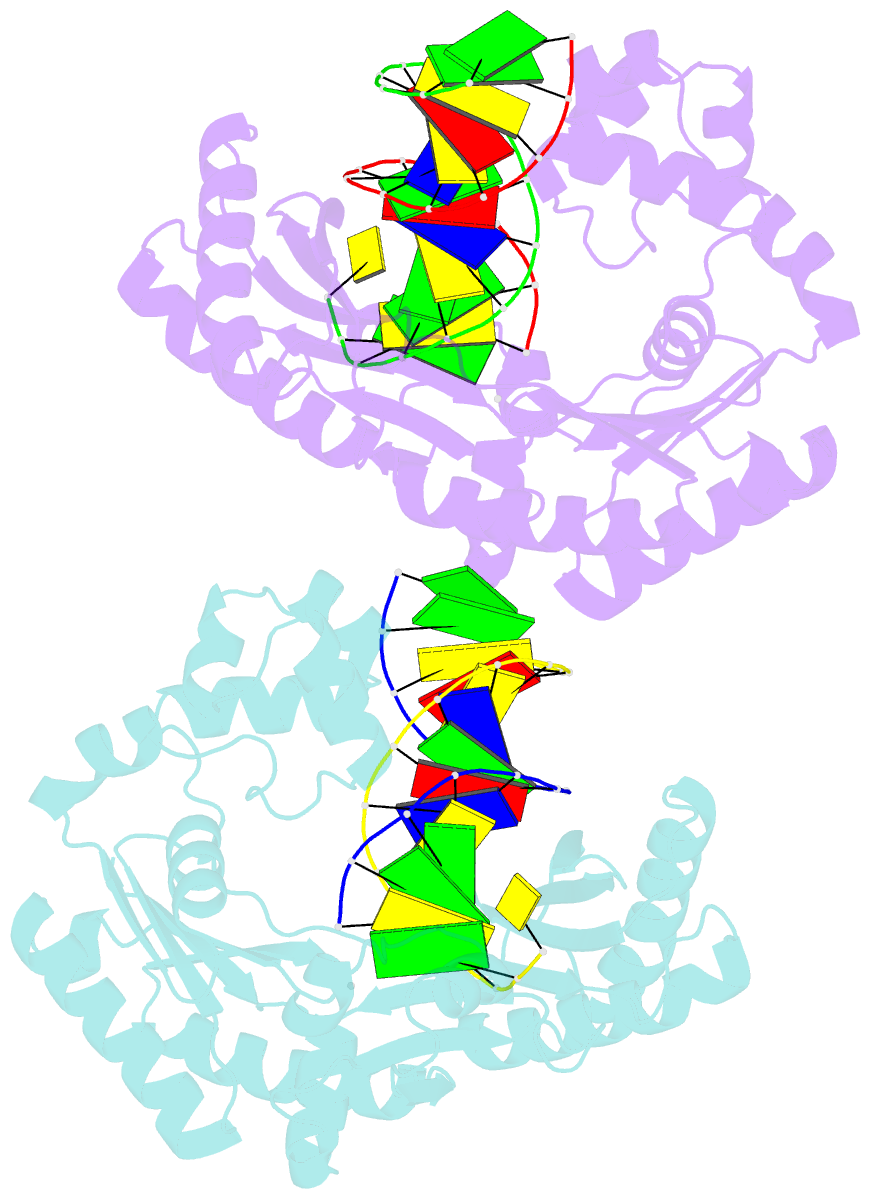
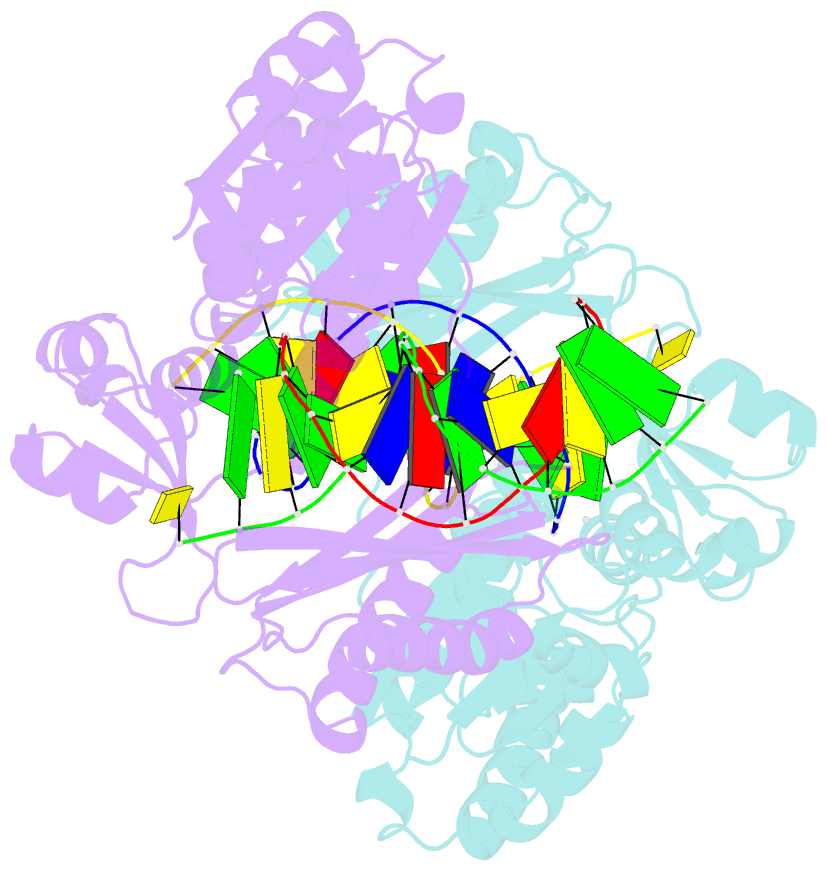
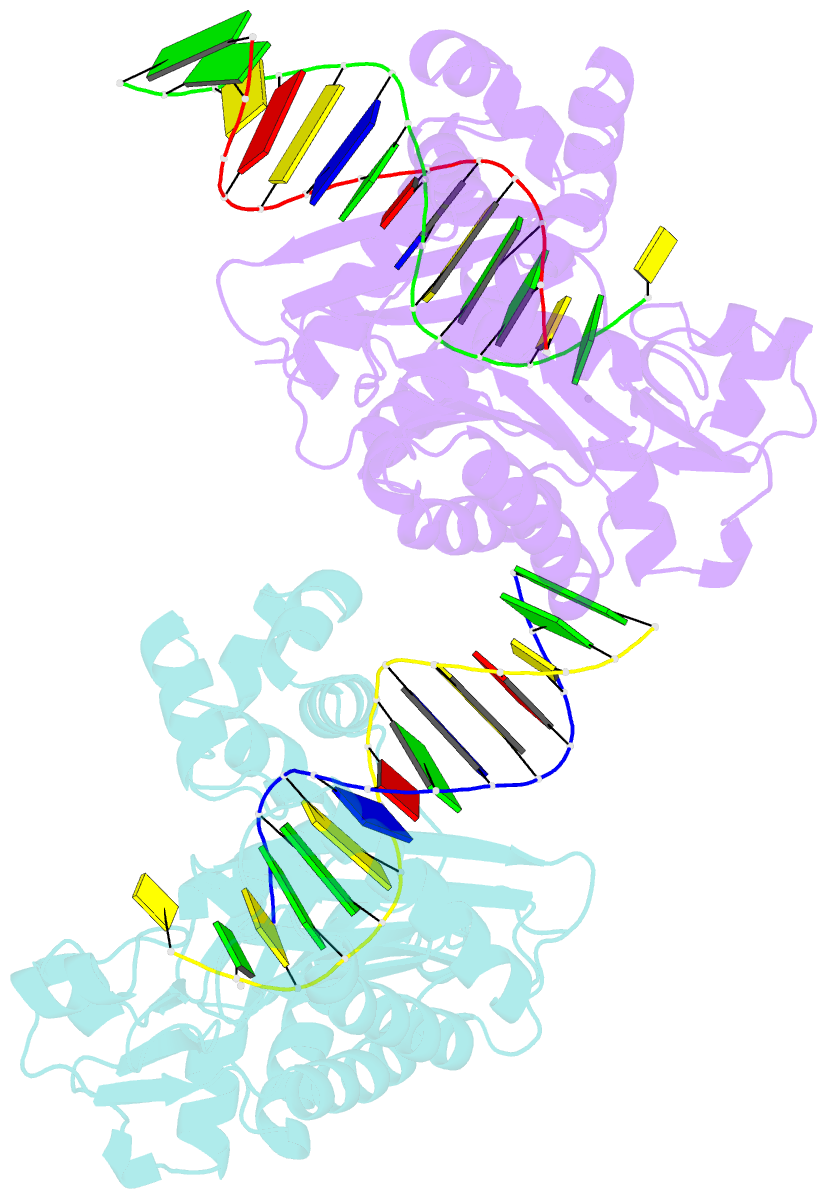
Summary information and primary citation
- PDB-id
- 4f4w; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (1.898 Å)
- Summary
- Y-family DNA polymerase chimera dbh-dpo4-dpo4 #1
- Reference
- Wilson RC, Jackson MA, Pata JD (2013): "Y-family polymerase conformation is a major determinant of fidelity and translesion specificity." Structure, 21, 20-31. doi: 10.1016/j.str.2012.11.005.
- Abstract
- Y-family polymerases help cells tolerate DNA damage by performing translesion synthesis opposite damaged DNA bases, yet they also have a high intrinsic error rate. We constructed chimeras of two closely related Y-family polymerases that display distinctly different activity profiles and found that the polypeptide linker that tethers the catalytic polymerase domain to the C-terminal DNA-binding domain is a major determinant of overall polymerase activity, nucleotide incorporation fidelity, and abasic site-bypass ability. Exchanging just 3 out of the 15 linker residues is sufficient to interconvert the polymerase activities tested. Crystal structures of four chimeras show that the conformation of the protein correlates with the identity of the interdomain linker sequence. Thus, residues that are more than 15 Å away from the active site are able to influence many aspects of polymerase activity by altering the relative orientations of the catalytic and DNA-binding domains.