Summary information and primary citation
- PDB-id
- 4g0u; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA-isomerase inhibitor
- Method
- X-ray (2.7 Å)
- Summary
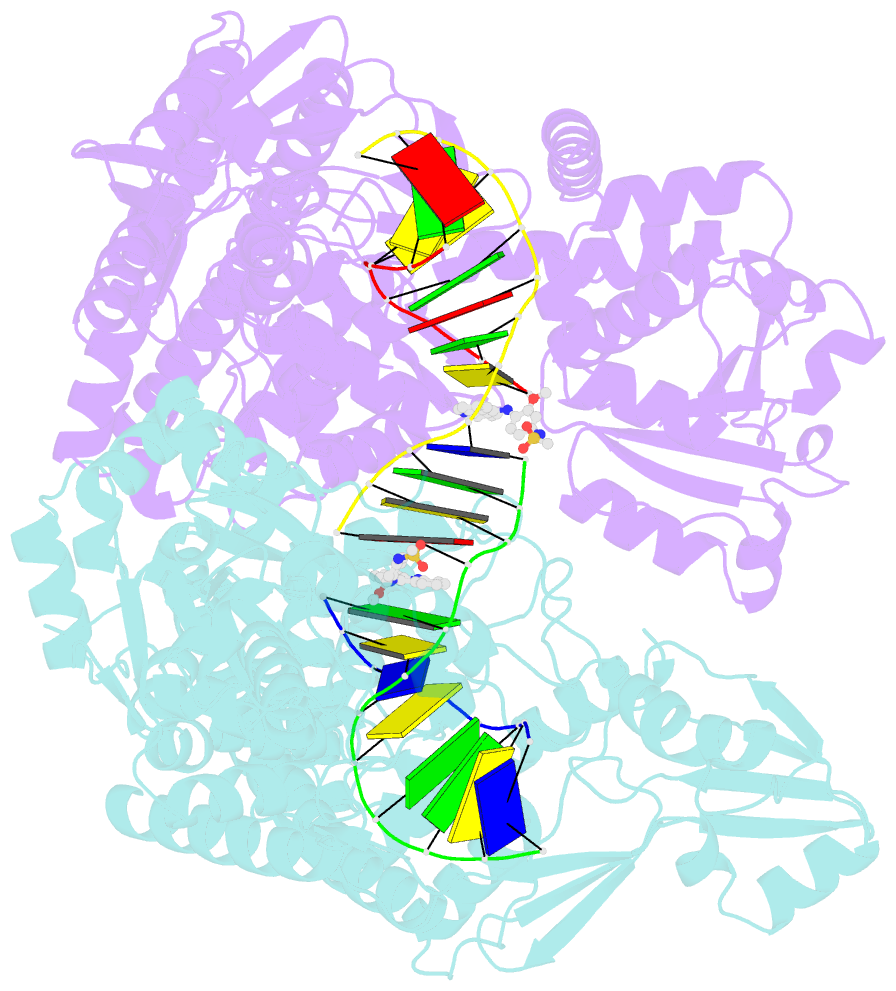
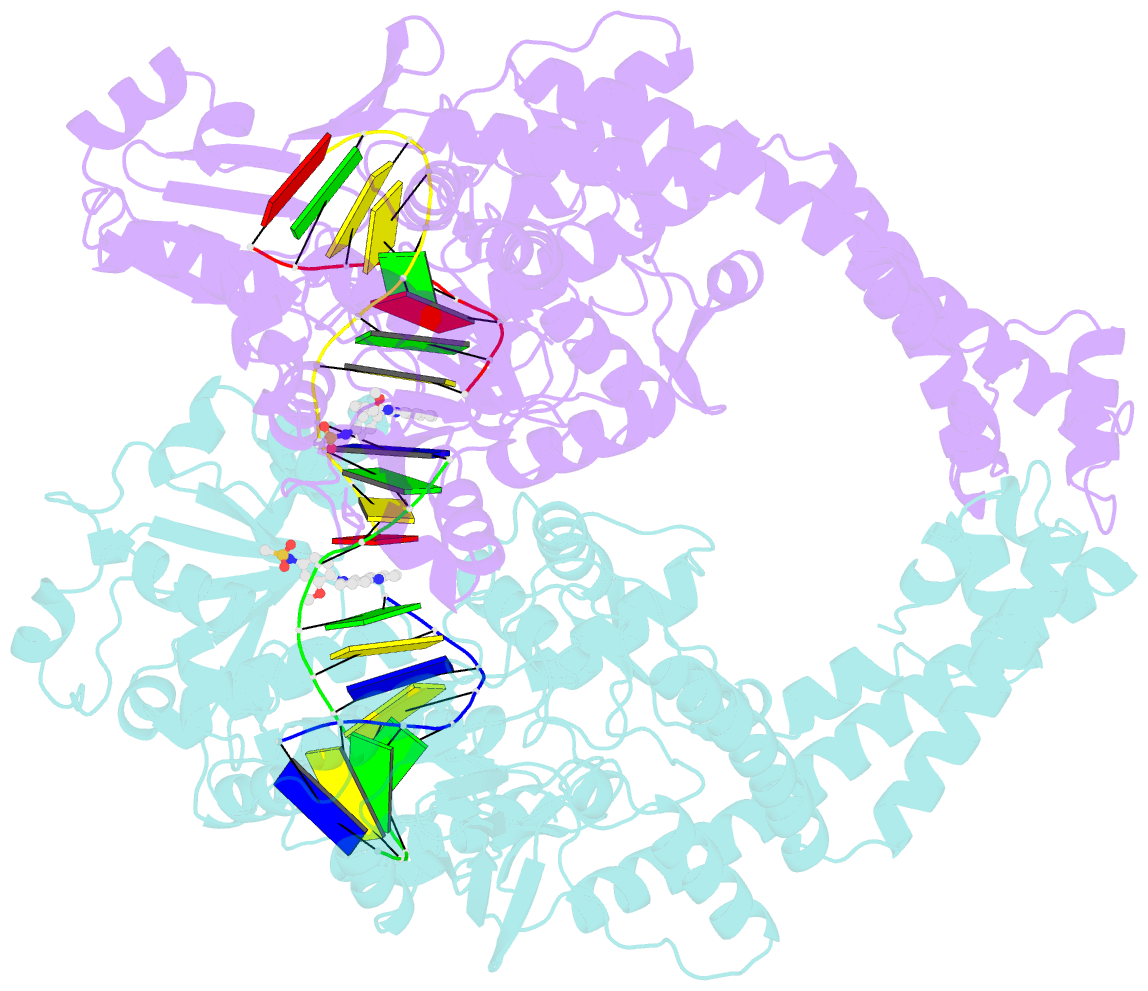
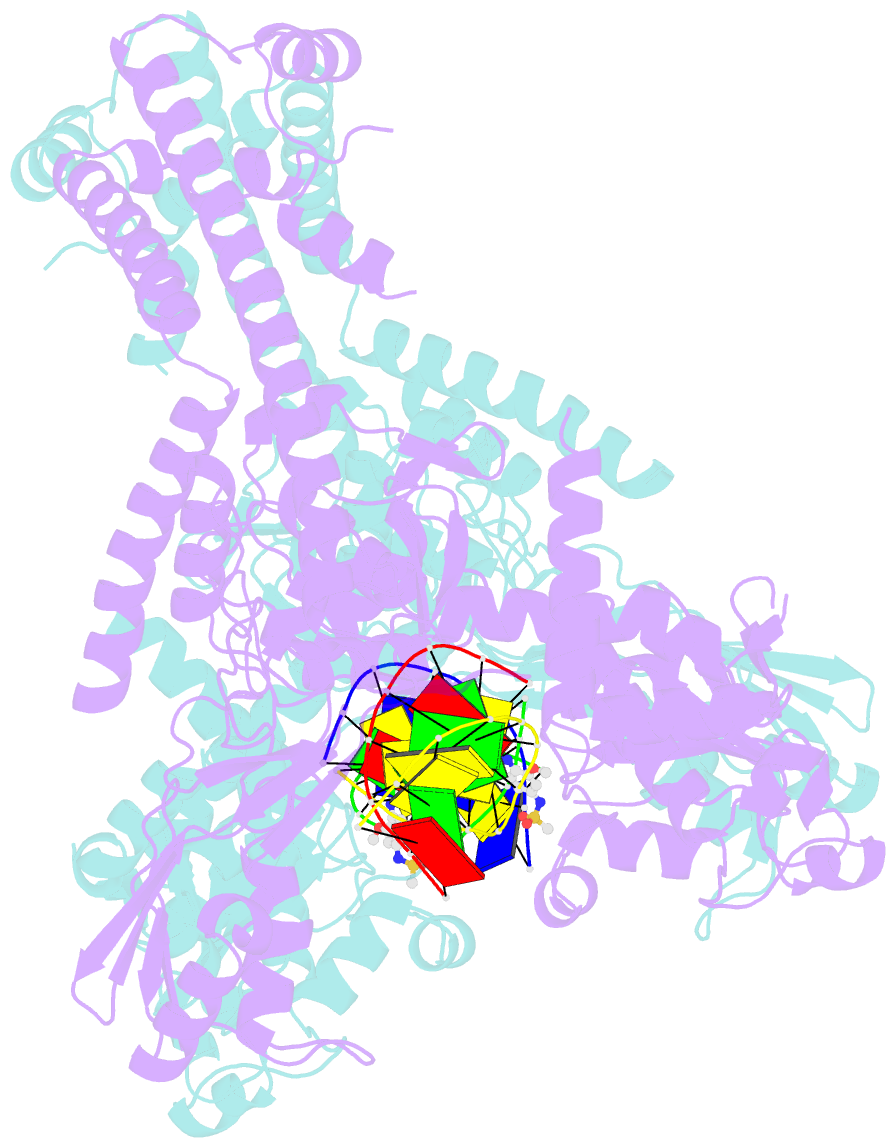
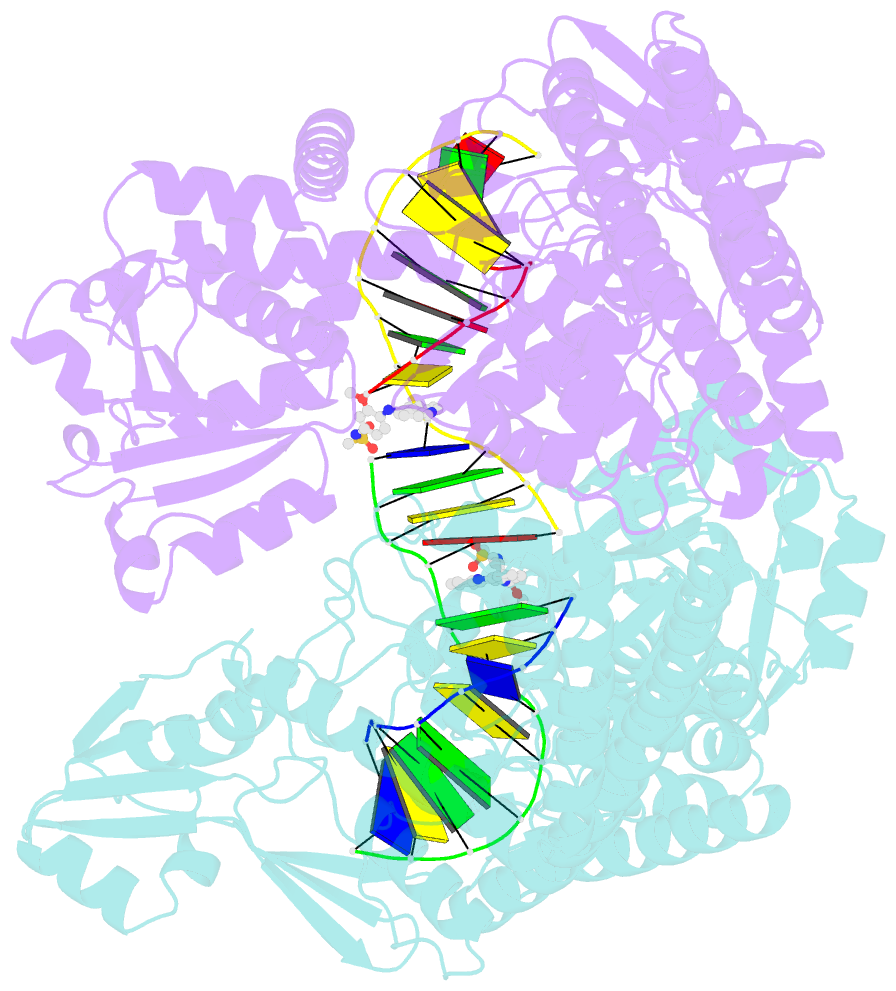
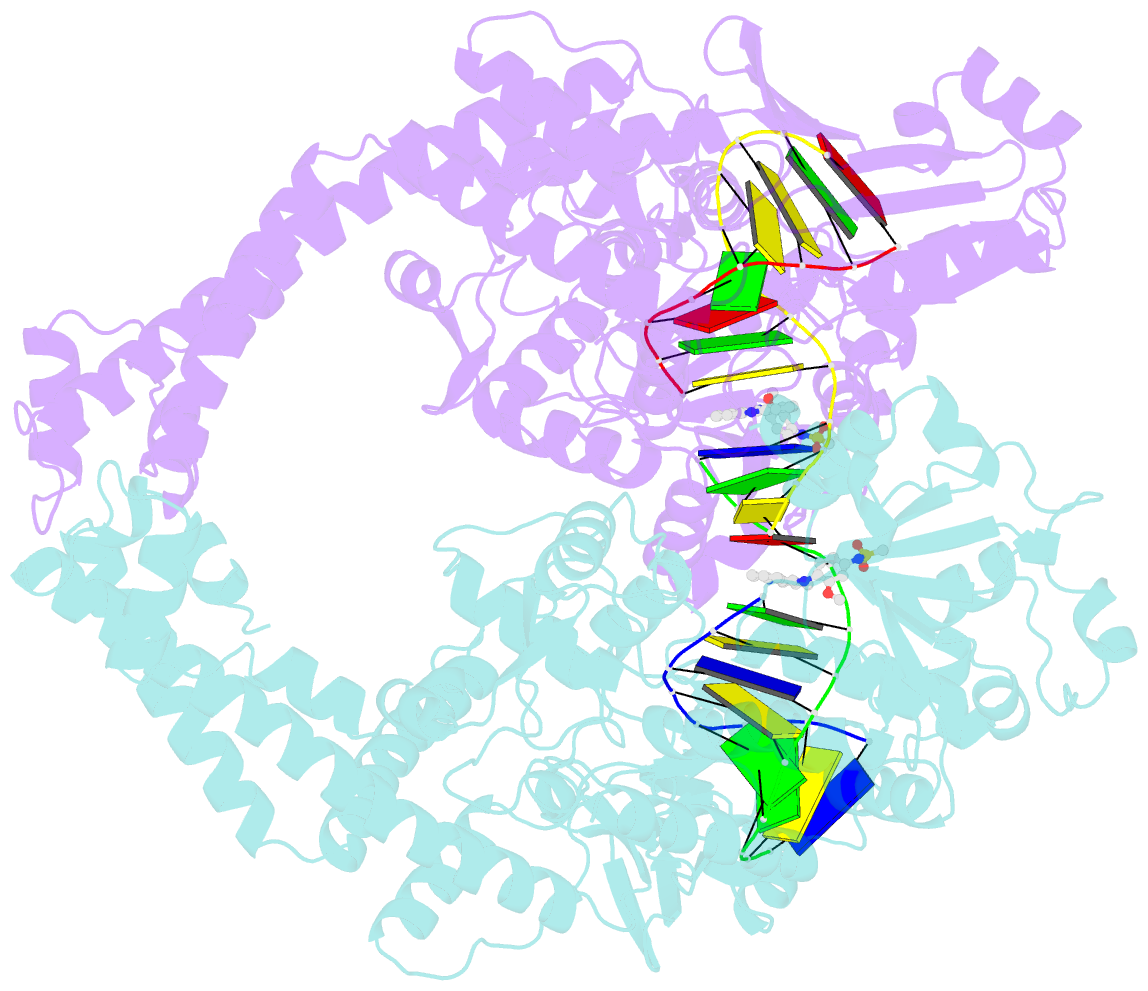
- Human topoisomerase iibeta in complex with DNA and amsacrine
- Reference
- Wu CC, Li YC, Wang YR, Li TK, Chan NL (2013): "On the structural basis and design guidelines for type II topoisomerase-targeting anticancer drugs." Nucleic Acids Res., 41, 10630-10640. doi: 10.1093/nar/gkt828.
- Abstract
- Type II topoisomerases (Top2s) alter DNA topology via the formation of an enzyme-DNA adduct termed cleavage complex, which harbors a transient double-strand break in one DNA to allow the passage of another. Agents targeting human Top2s are clinically active anticancer drugs whose trapping of Top2-mediated DNA breakage effectively induces genome fragmentation and cell death. To understand the structural basis of this drug action, we previously determined the structure of human Top2 β-isoform forming a cleavage complex with the drug etoposide and DNA, and described the insertion of drug into DNA cleavage site and drug-induced decoupling of catalytic groups. By developing a post-crystallization drug replacement procedure that simplifies structural characterization of drug-stabilized cleavage complexes, we have extended the analysis toward other structurally distinct drugs, m-AMSA and mitoxantrone. Besides the expected drug intercalation, a switch in ribose puckering in the 3'-nucleotide of the cleavage site was robustly observed in the new structures, representing a new mechanism for trapping the Top2 cleavage complex. Analysis of drug-binding modes and the conformational landscapes of the drug-binding pockets provide rationalization of the drugs' structural-activity relationships and explain why Top2 mutants exhibit differential effects toward each drug. Drug design guidelines were proposed to facilitate the development of isoform-specific Top2-targeting anticancer agents.