Summary information and primary citation
- PDB-id
- 4gop; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (3.1 Å)
- Summary
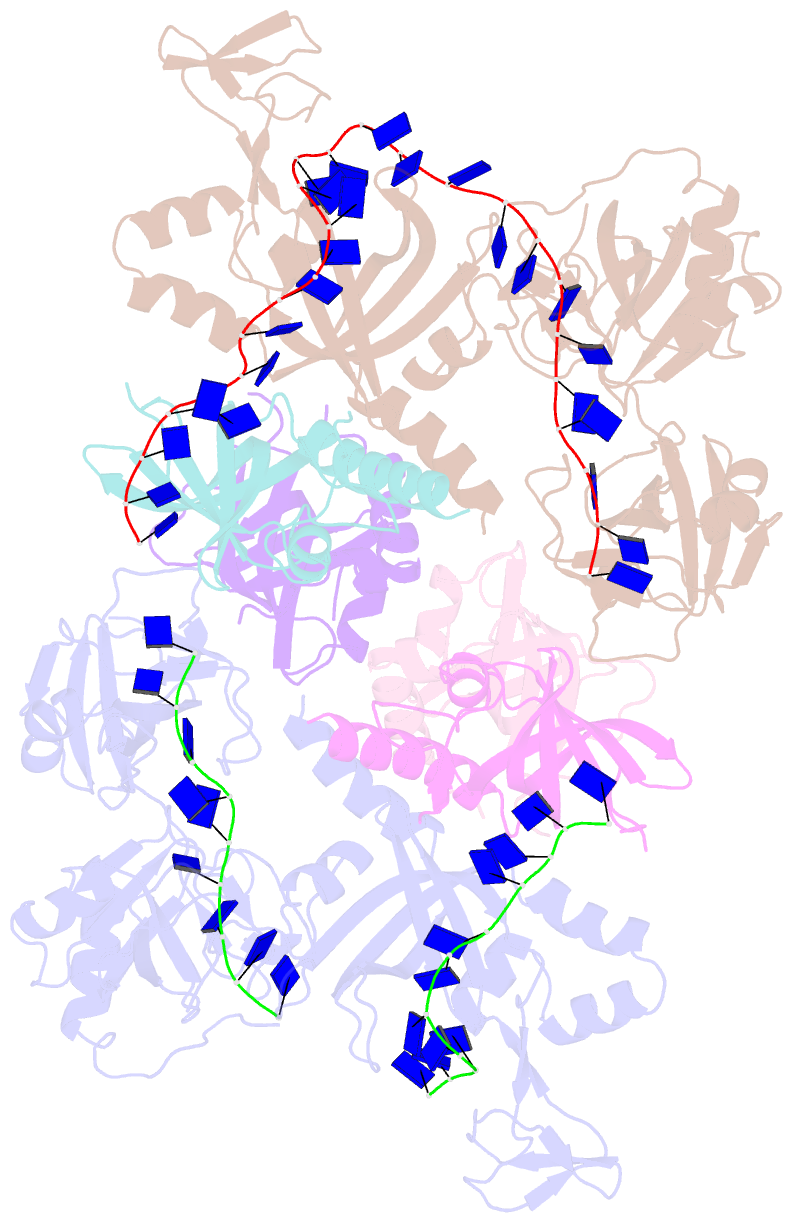
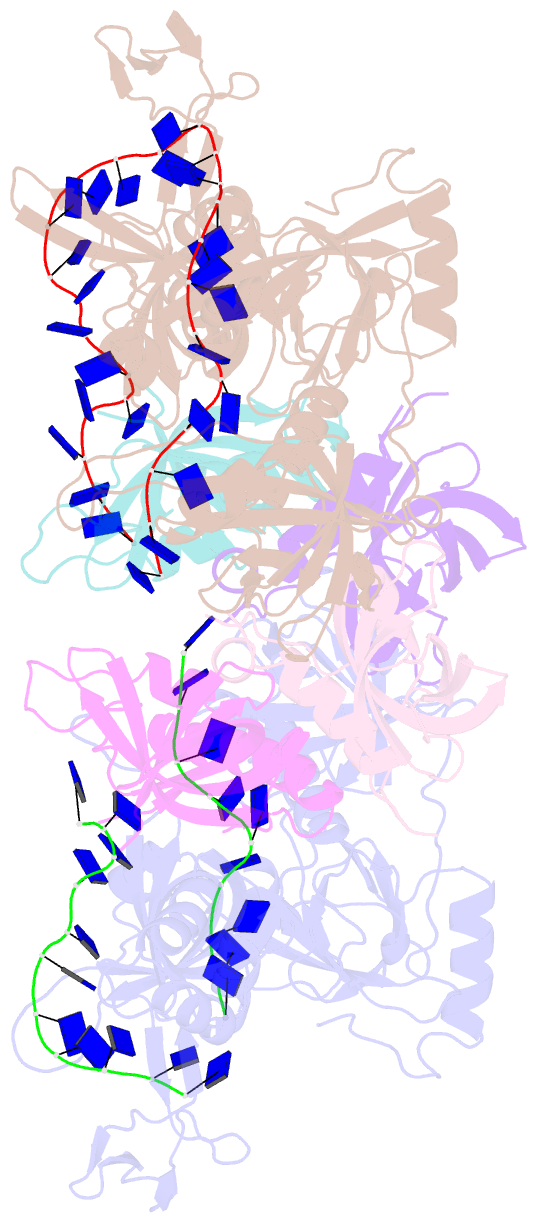
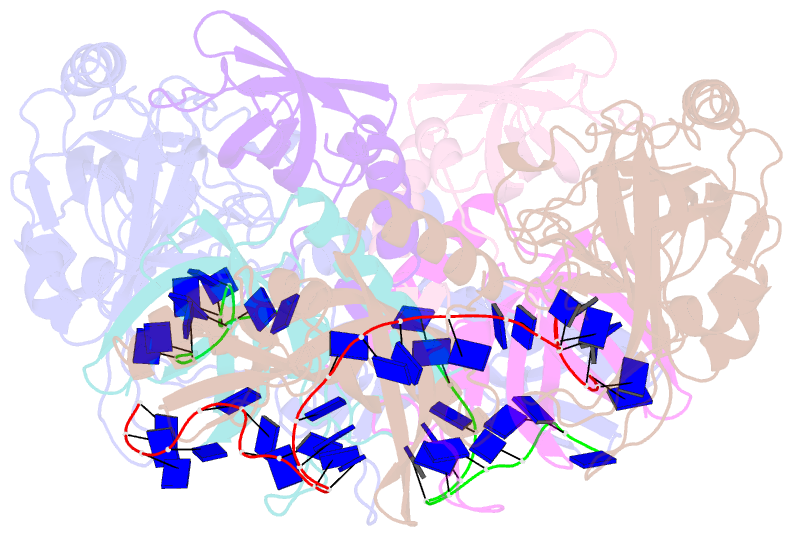
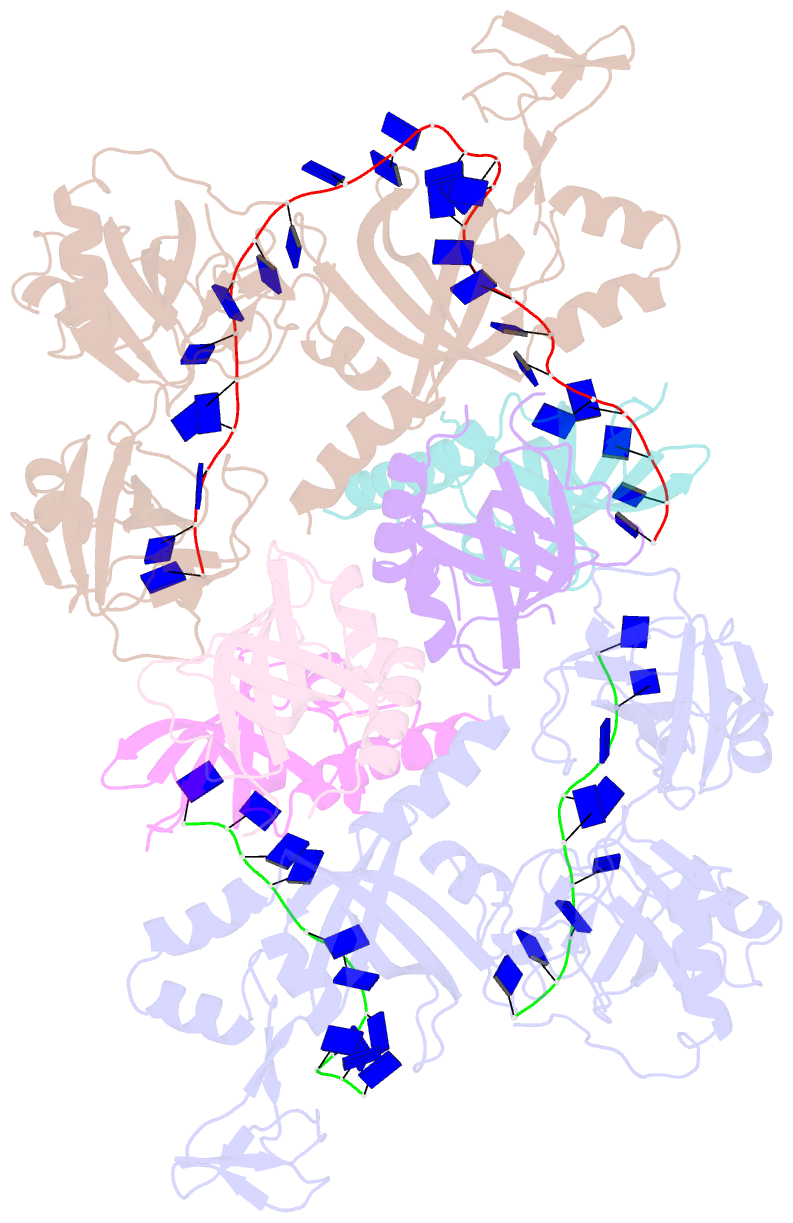
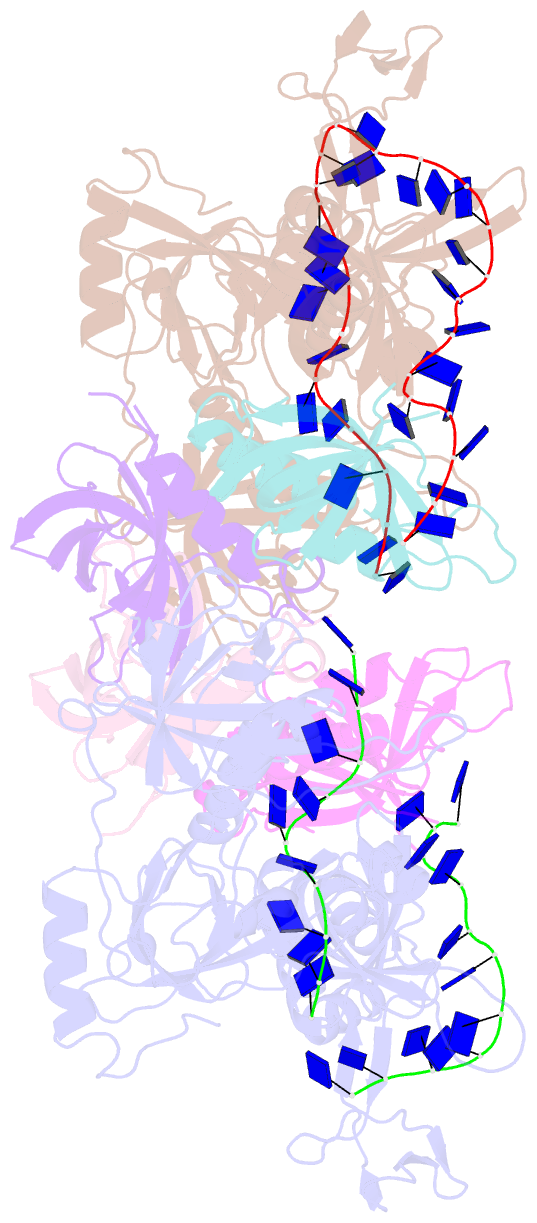
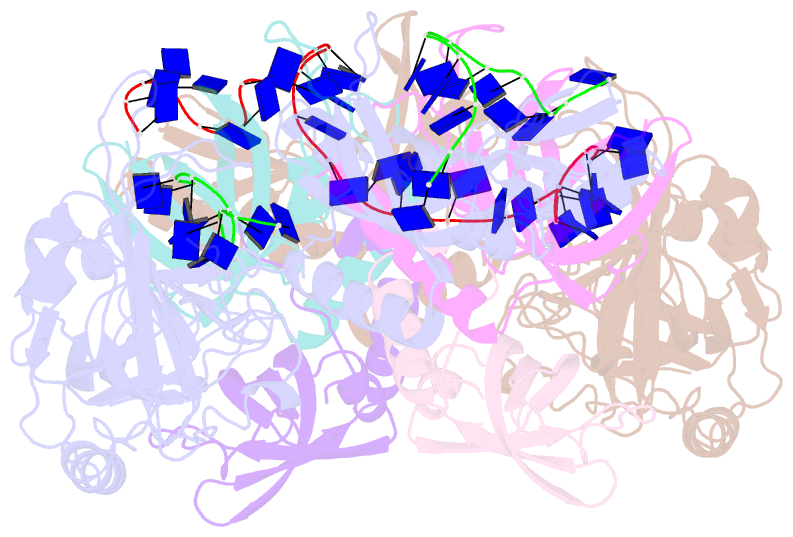
- Structure and conformational change of a replication protein a heterotrimer bound to ssDNA
- Reference
- Fan J, Pavletich NP (2012): "Structure and conformational change of a replication protein A heterotrimer bound to ssDNA." Genes Dev., 26, 2337-2347. doi: 10.1101/gad.194787.112.
- Abstract
- Replication protein A (RPA) is the main eukaryotic ssDNA-binding protein with essential roles in DNA replication, recombination, and repair. RPA maintains the DNA as single-stranded and also interacts with other DNA-processing proteins, coordinating their assembly and disassembly on DNA. RPA binds to ssDNA in two conformational states with opposing affinities for DNA and proteins. The RPA-protein interactions are compatible with a low DNA affinity state that involves DNA-binding domain A (DBD-A) and DBD-B but not with the high DNA affinity state that additionally engages DBD-C and DBD-D. The structure of the high-affinity RPA-ssDNA complex reported here shows a compact quaternary structure held together by a four-way interface between DBD-B, DBD-C, the intervening linker (BC linker), and ssDNA. The BC linker binds into the DNA-binding groove of DBD-B, mimicking DNA. The associated conformational change and partial occlusion of the DBD-A-DBA-B protein-protein interaction site establish a mechanism for the allosteric coupling of RPA-DNA and RPA-protein interactions.