Summary information and primary citation
- PDB-id
- 4hsb; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.9 Å)
- Summary
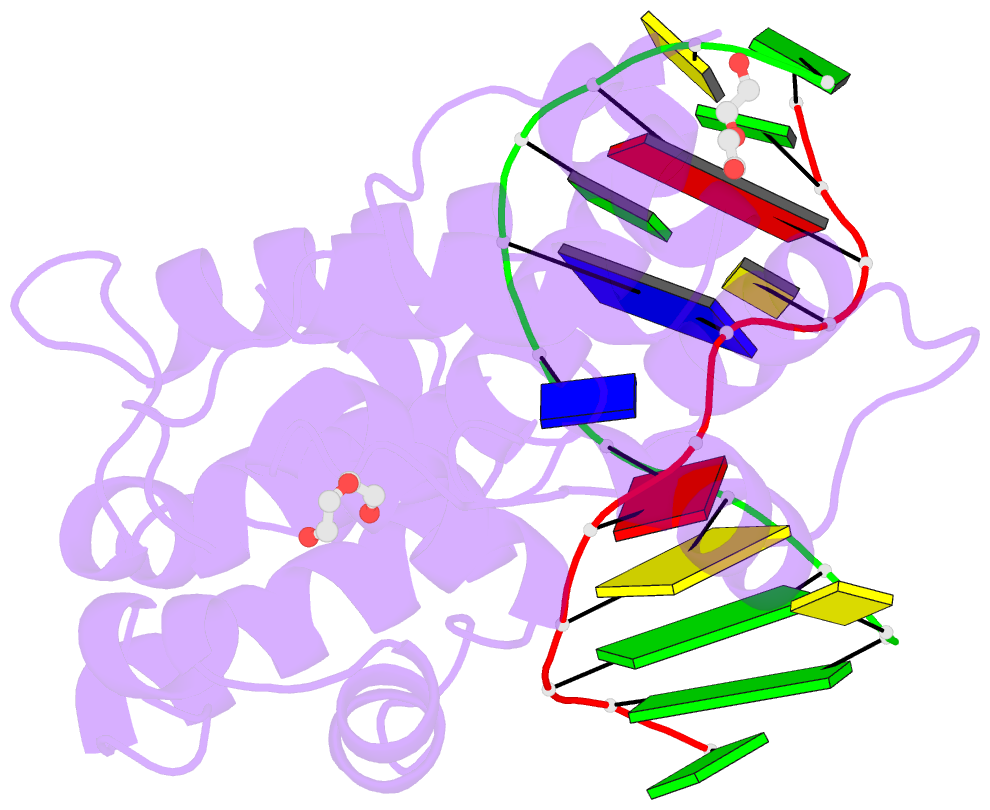
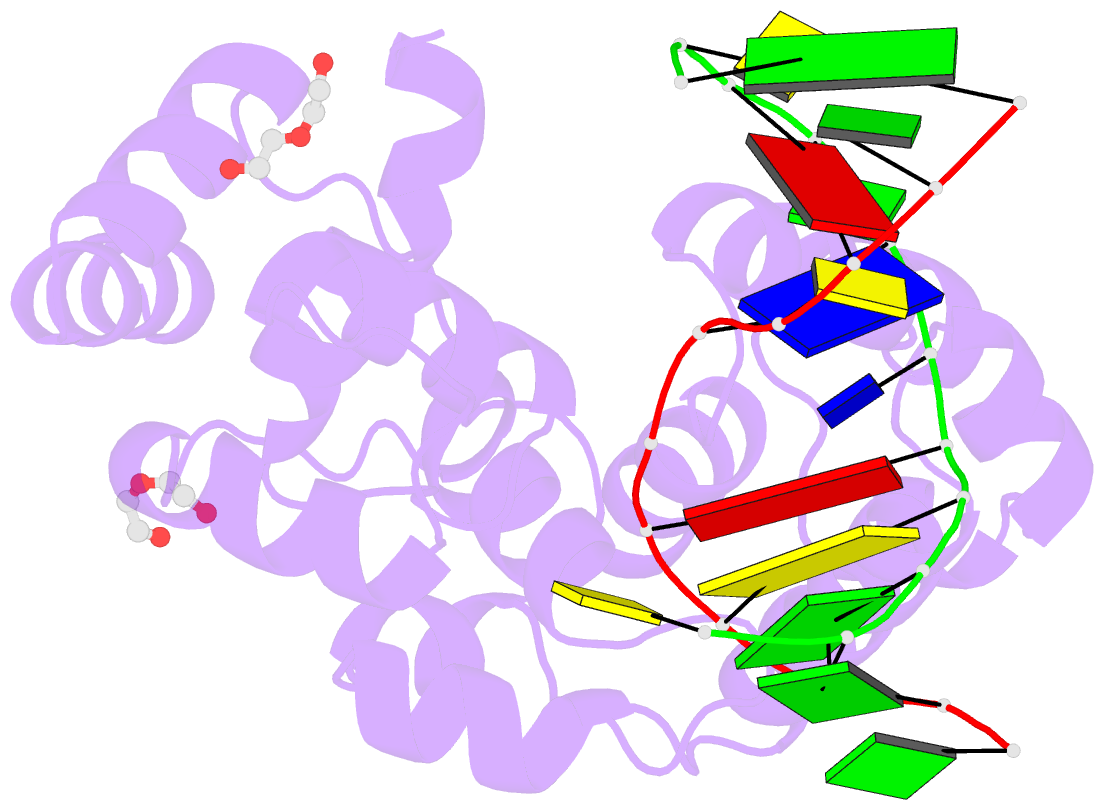
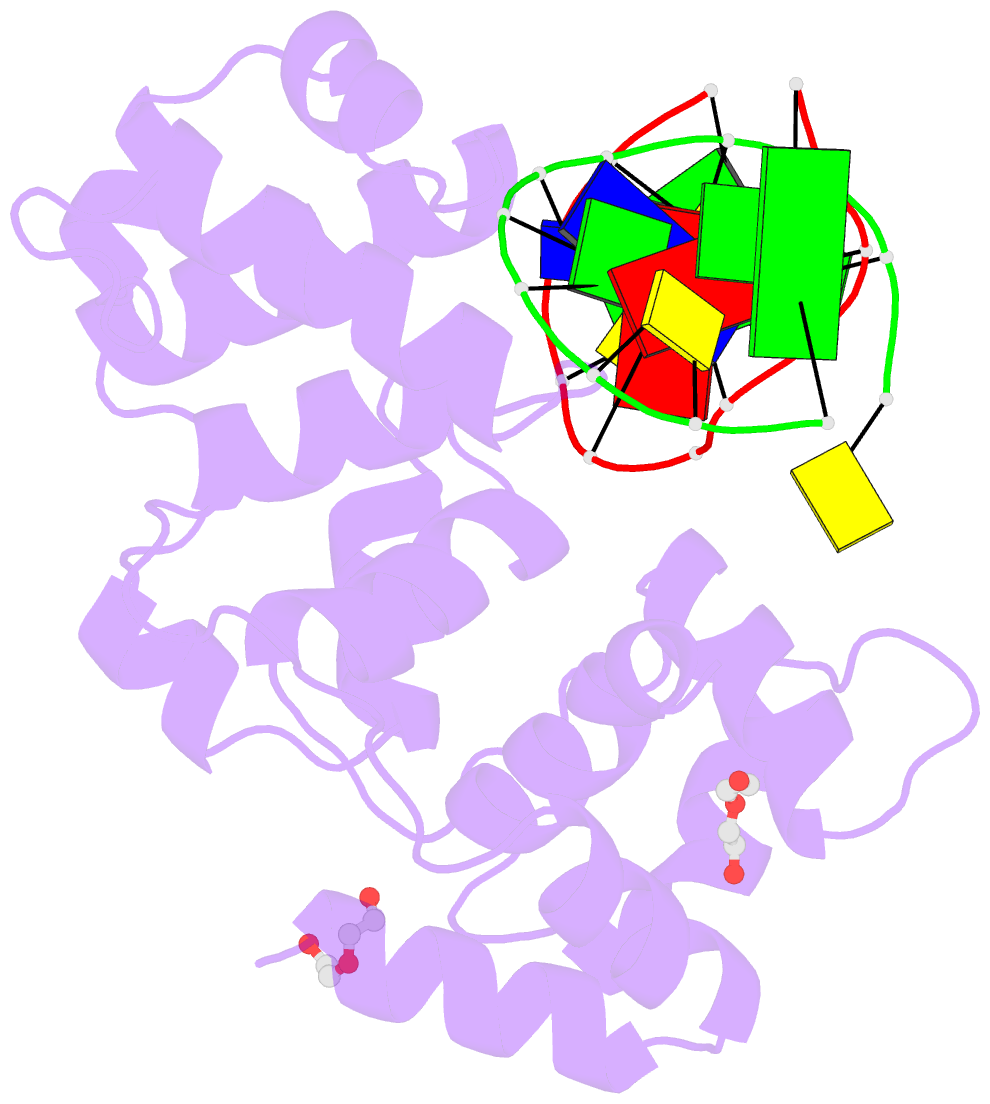
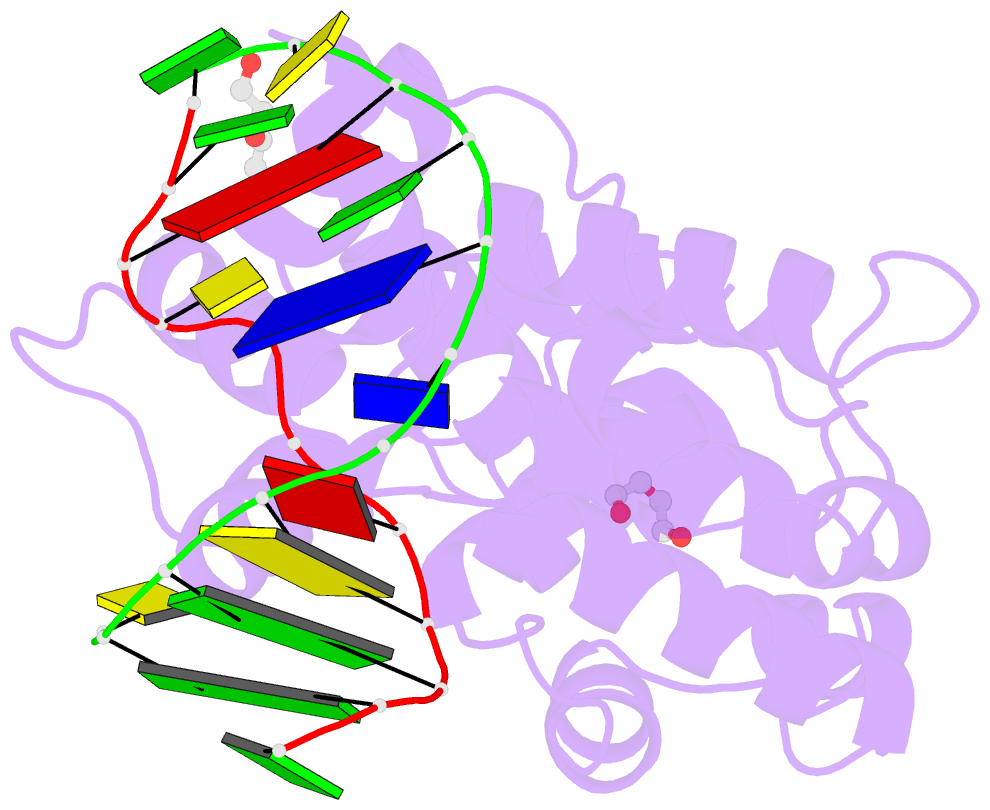
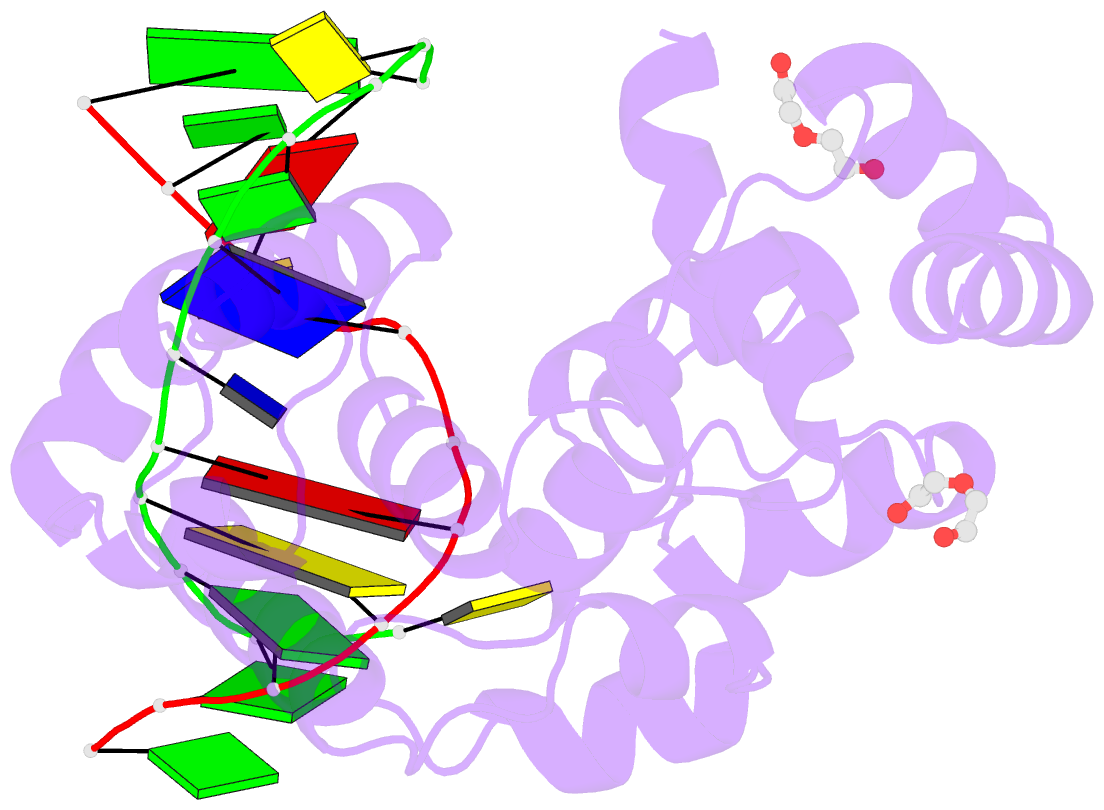
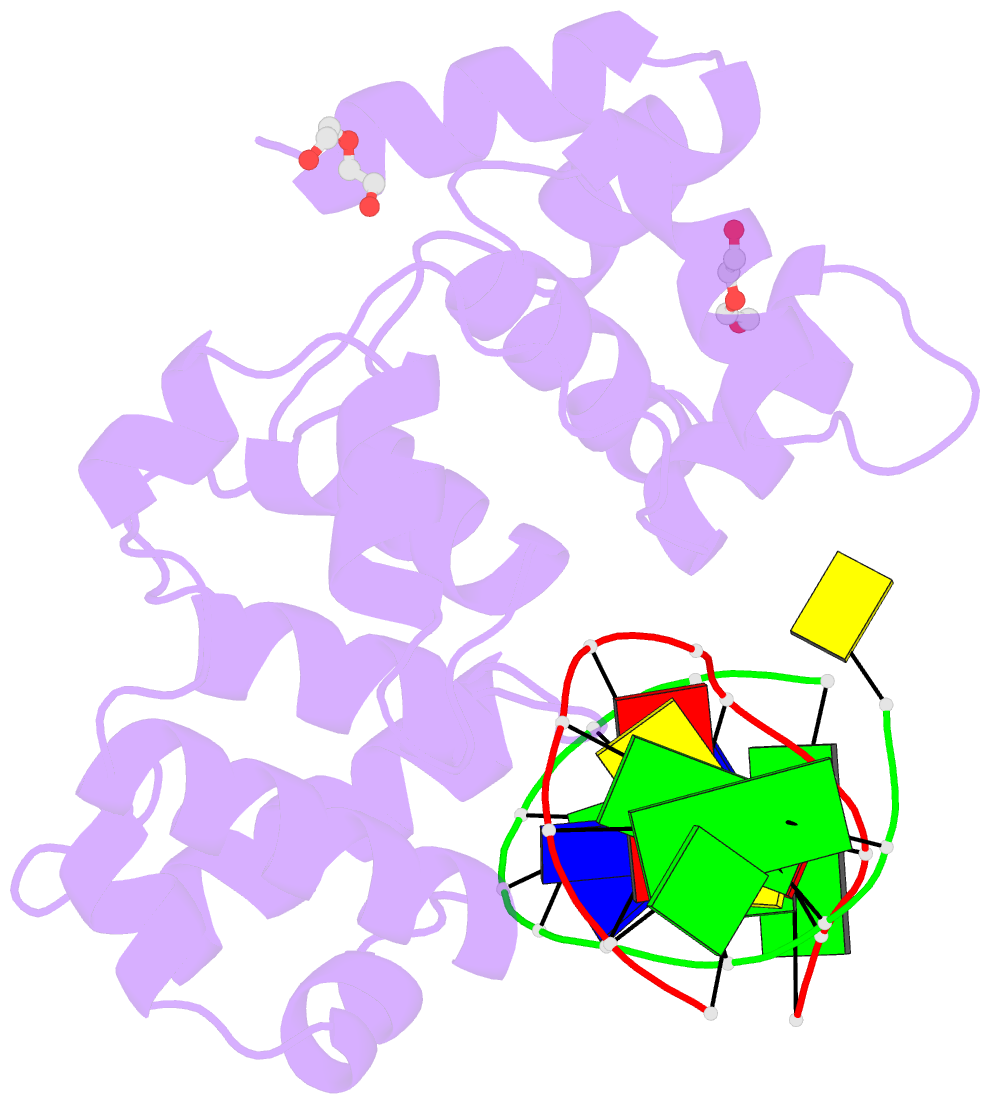
- S. pombe 3-methyladenine DNA glycosylase-like protein mag2 bound to damaged DNA
- Reference
- Adhikary S, Cato MC, McGary KL, Rokas A, Eichman BF (2013): "Non-productive DNA damage binding by DNA glycosylase-like protein Mag2 from Schizosaccharomyces pombe." Dna Repair, 12, 196-204. doi: 10.1016/j.dnarep.2012.12.001.
- Abstract
- Schizosaccharomyces pombe contains two paralogous proteins, Mag1 and Mag2, related to the helix-hairpin-helix (HhH) superfamily of alkylpurine DNA glycosylases from yeast and bacteria. Phylogenetic analysis of related proteins from four Schizosaccharomyces and other fungal species shows that the Mag1/Mag2 duplication is unique to the genus Schizosaccharomyces and most likely occurred in its ancestor. Mag1 excises N3- and N7-alkylguanines and 1,N(6)-ethenoadenine from DNA, whereas Mag2 has been reported to have no detectible alkylpurine base excision activity despite high sequence and active site similarity to Mag1. To understand this discrepancy we determined the crystal structure of Mag2 bound to abasic DNA and compared it to our previously determined Mag1-DNA structure. In contrast to Mag1, Mag2 does not flip the abasic moiety into the active site or stabilize the DNA strand 5' to the lesion, suggesting that it is incapable of forming a catalytically competent protein-DNA complex. Subtle differences in Mag1 and Mag2 interactions with the DNA duplex illustrate how Mag2 can stall at damage sites without fully engaging the lesion. We tested our structural predictions by mutational analysis of base excision and found a single amino acid responsible at least in part for Mag2's lack of activity. Substitution of Mag2 Asp56, which caps the helix at the base of the DNA intercalation loop, with the corresponding serine residue in Mag1 endows Mag2 with ɛA excision activity comparable to Mag1. This work provides novel insight into the chemical and physical determinants by which the HhH glycosylases engage DNA in a catalytically productive manner.