Summary information and primary citation
- PDB-id
- 4ht8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.9 Å)
- Summary
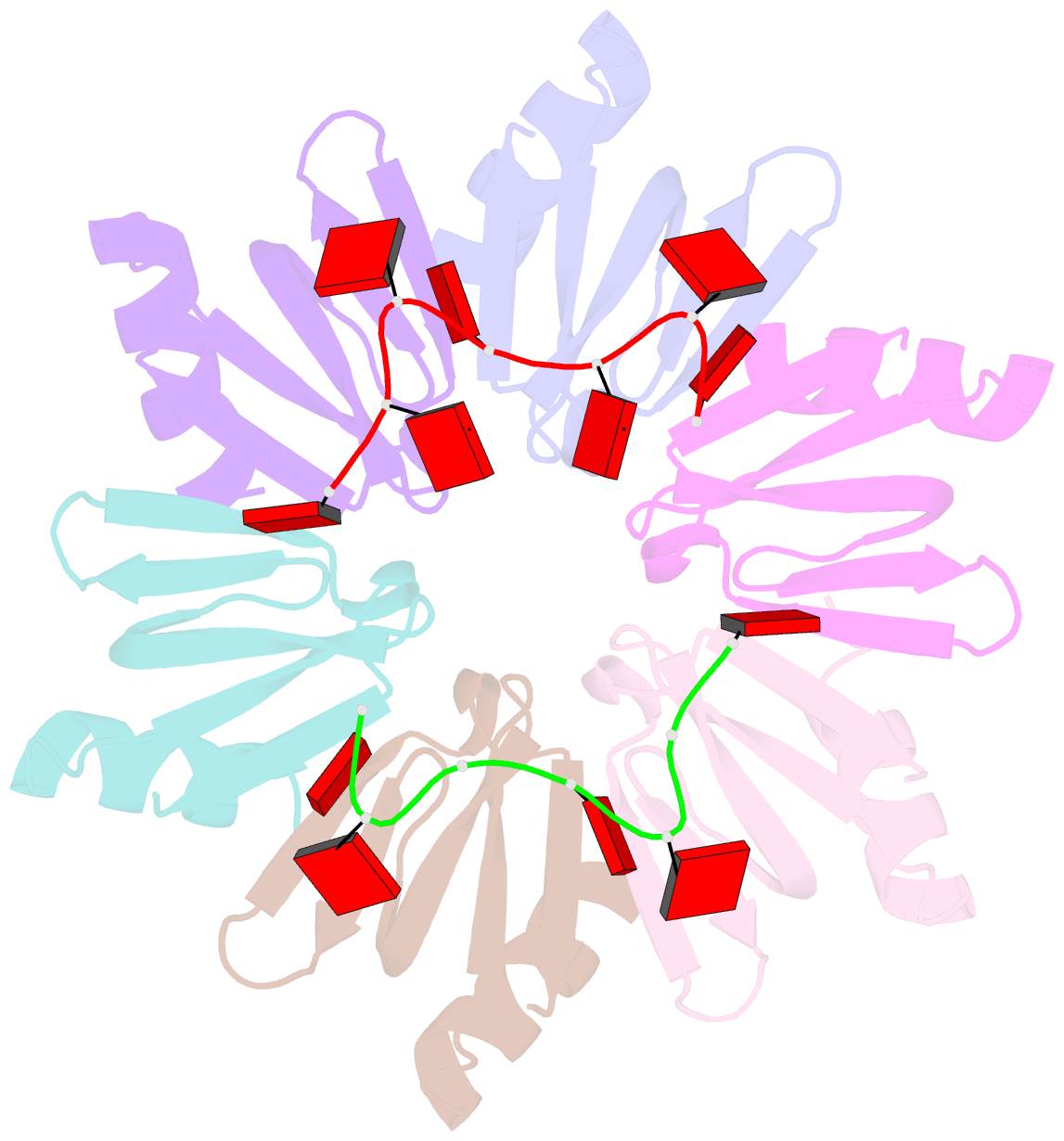
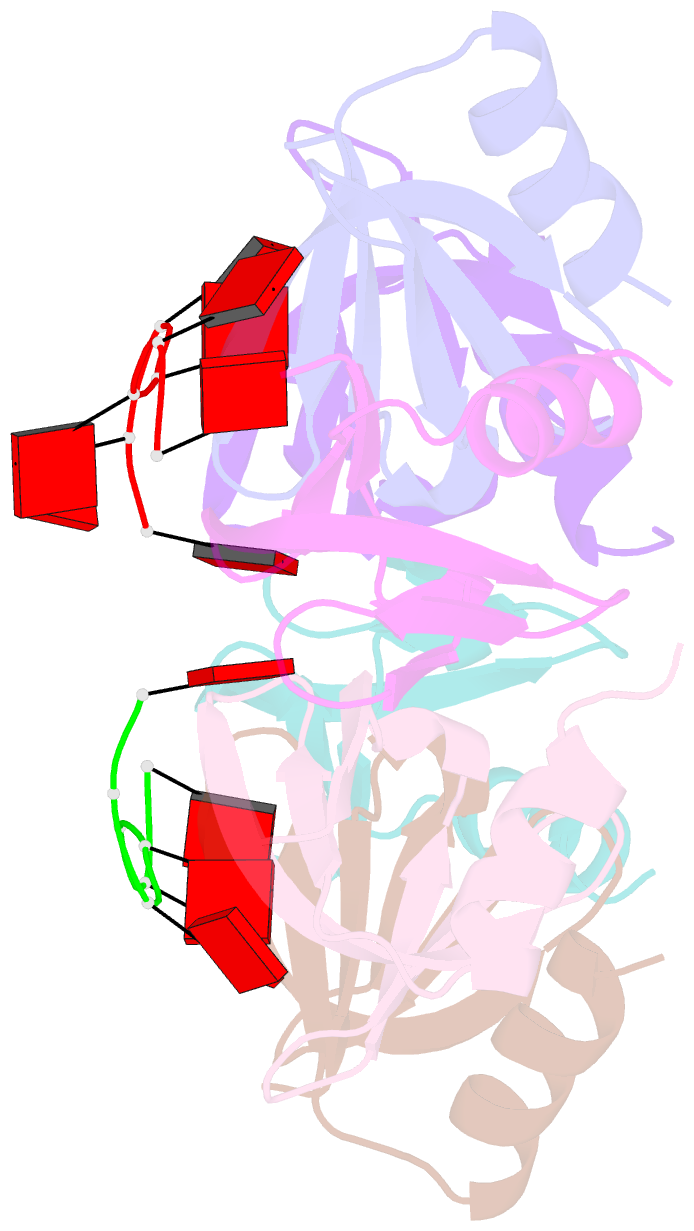
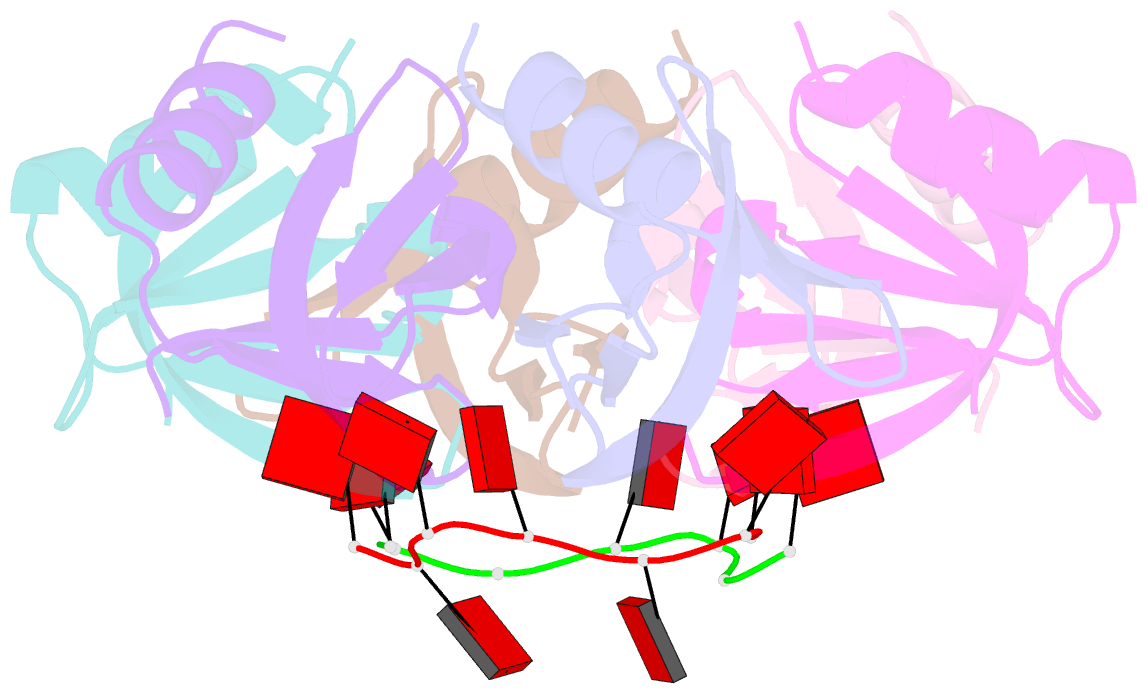
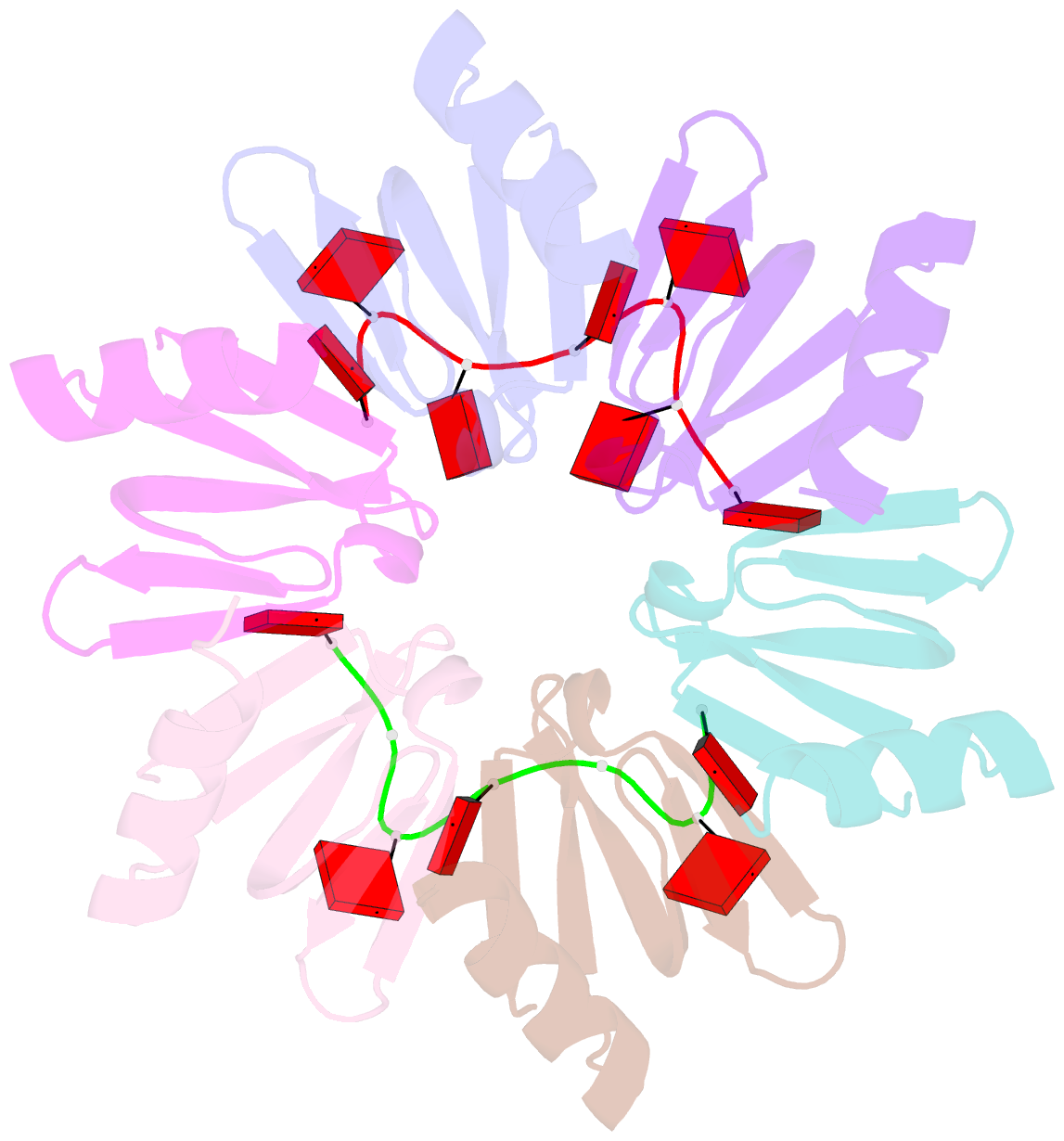
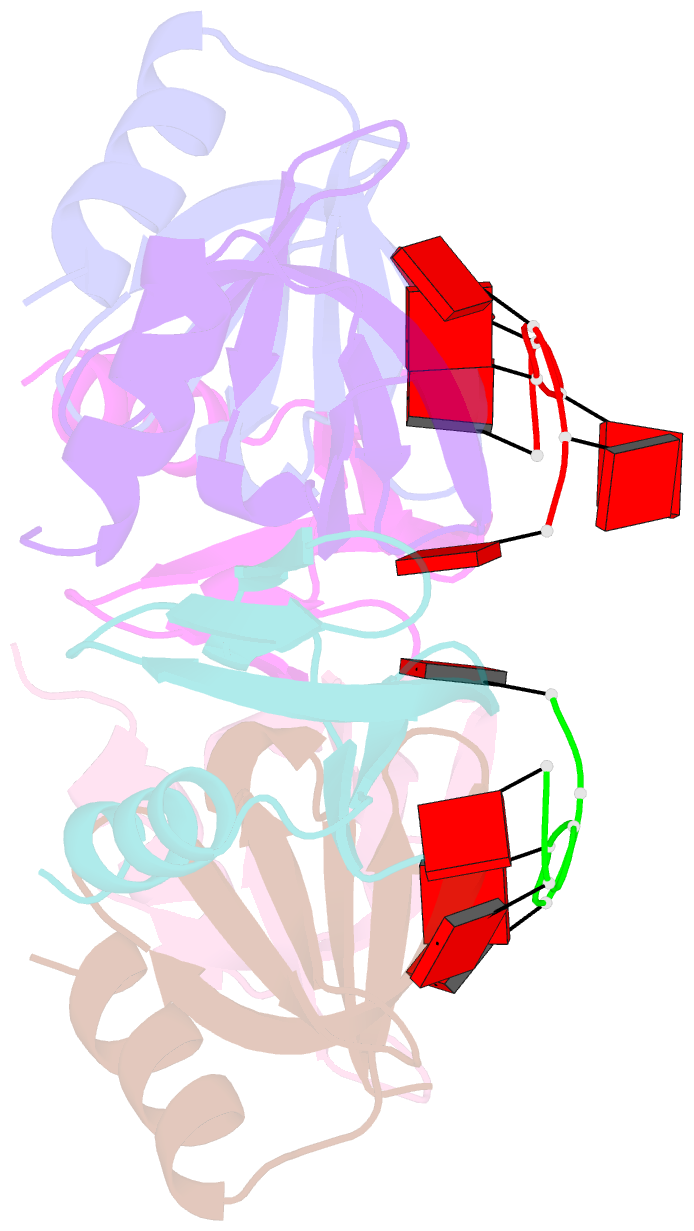
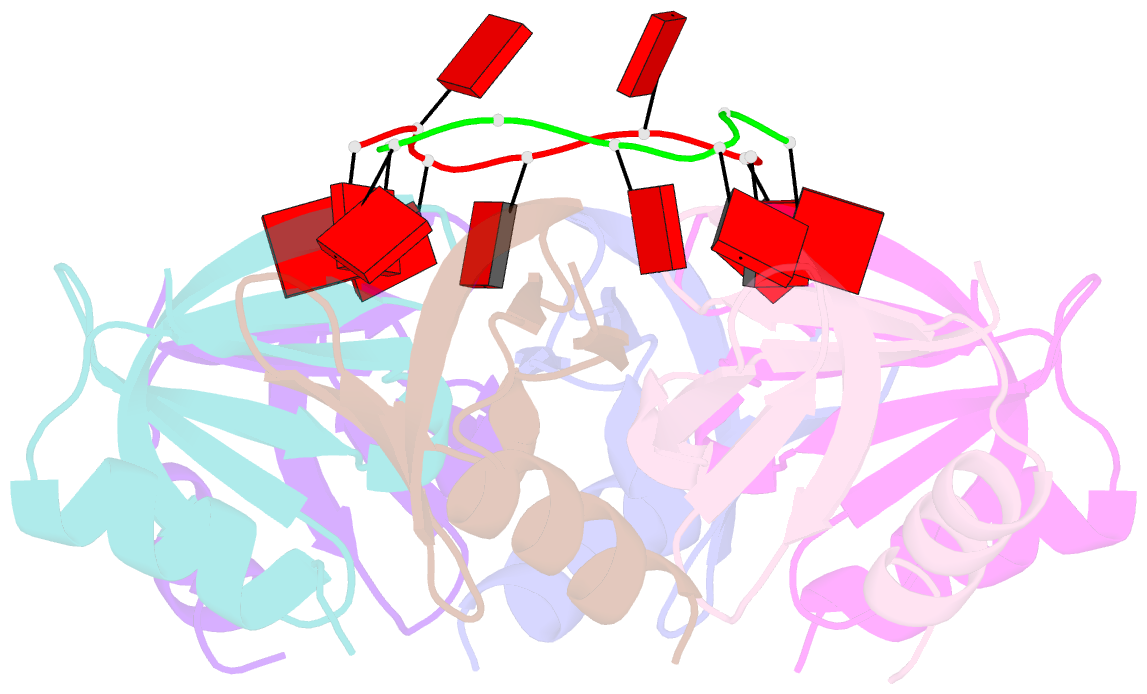
- Crystal structure of e coli hfq bound to poly(a) a7
- Reference
- Wang W, Wang L, Wu J, Gong Q, Shi Y (2013): "Hfq-bridged ternary complex is important for translation activation of rpoS by DsrA." Nucleic Acids Res., 41, 5938-5948. doi: 10.1093/nar/gkt276.
- Abstract
- The rpoS mRNA, which encodes the master regulator σ(S) of general stress response, requires Hfq-facilitated base pairing with DsrA small RNA for efficient translation at low temperatures. It has recently been proposed that one mechanism underlying Hfq action is to bridge a transient ternary complex by simultaneously binding to rpoS and DsrA. However, no structural evidence of Hfq simultaneously bound to different RNAs has been reported. We detected simultaneous binding of Hfq to rpoS and DsrA fragments. Crystal structures of AU6A•Hfq•A7 and Hfq•A7 complexes were resolved using 1.8- and 1.9-Å resolution, respectively. Ternary complex has been further verified in solution by NMR. In vivo, activation of rpoS translation requires intact Hfq, which is capable of bridging rpoS and DsrA simultaneously into ternary complex. This ternary complex possibly corresponds to a meta-stable transition state in Hfq-facilitated small RNA-mRNA annealing process.