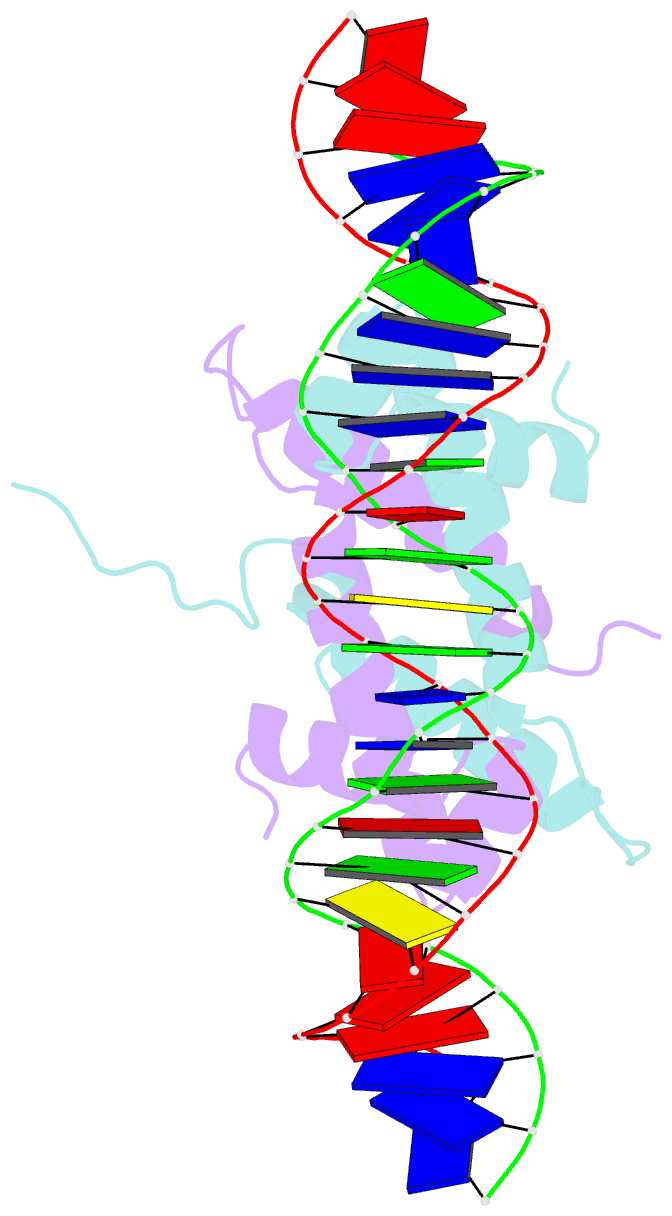
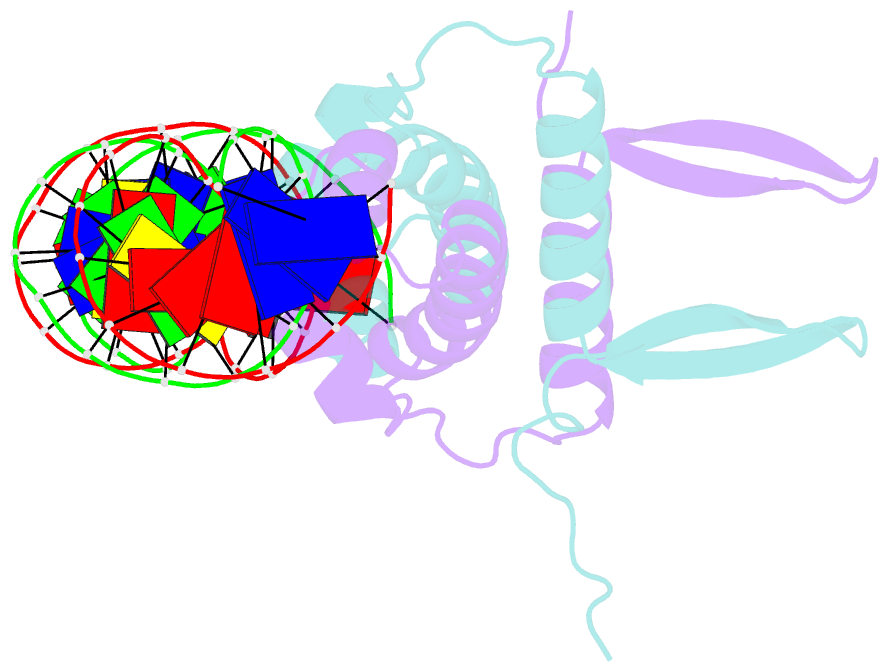
Summary information and primary citation
- PDB-id
- 4ihw; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.7 Å)
- Summary
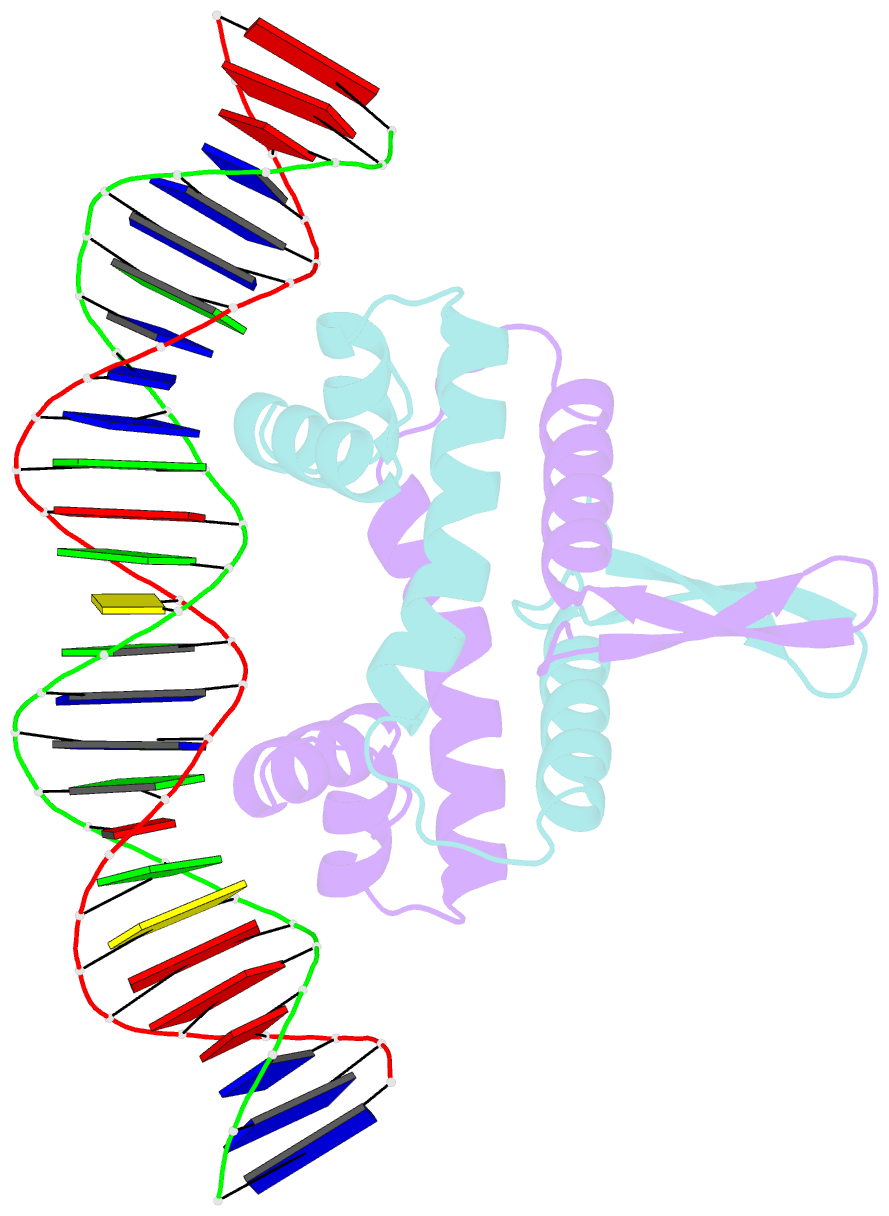
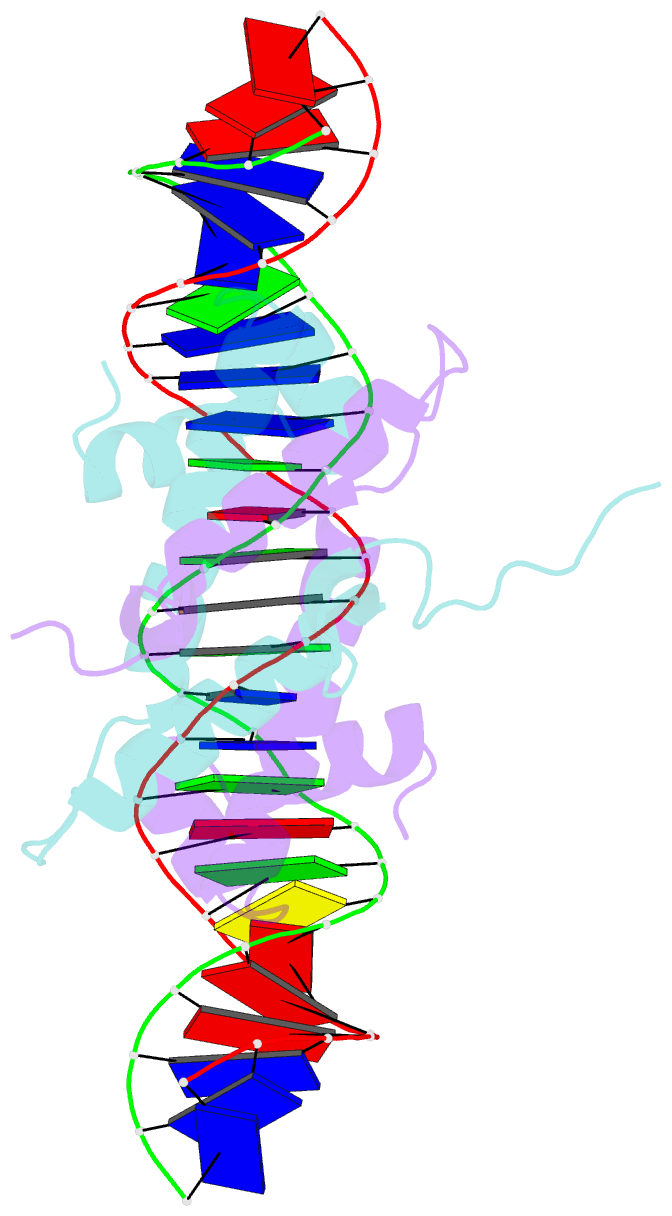
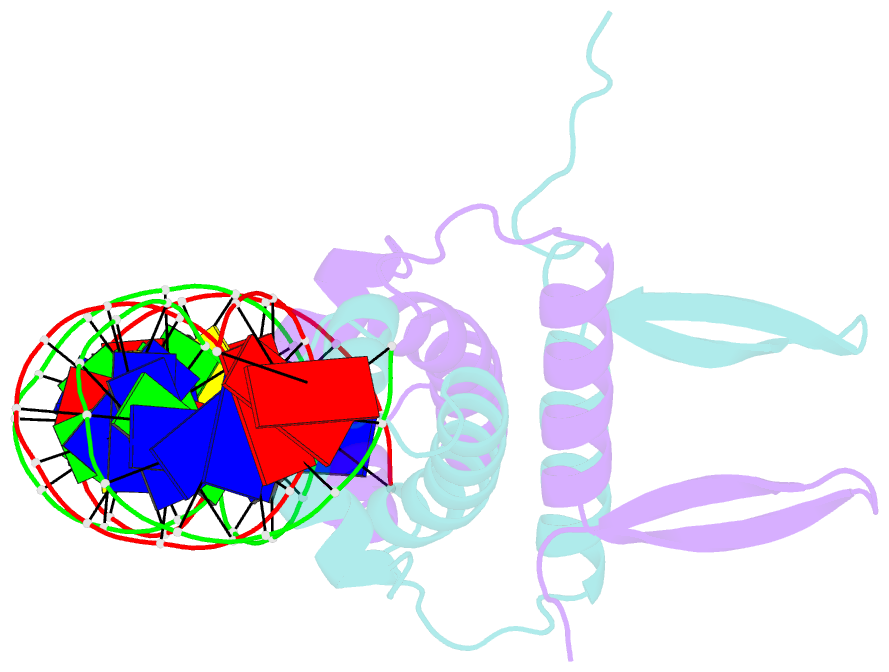
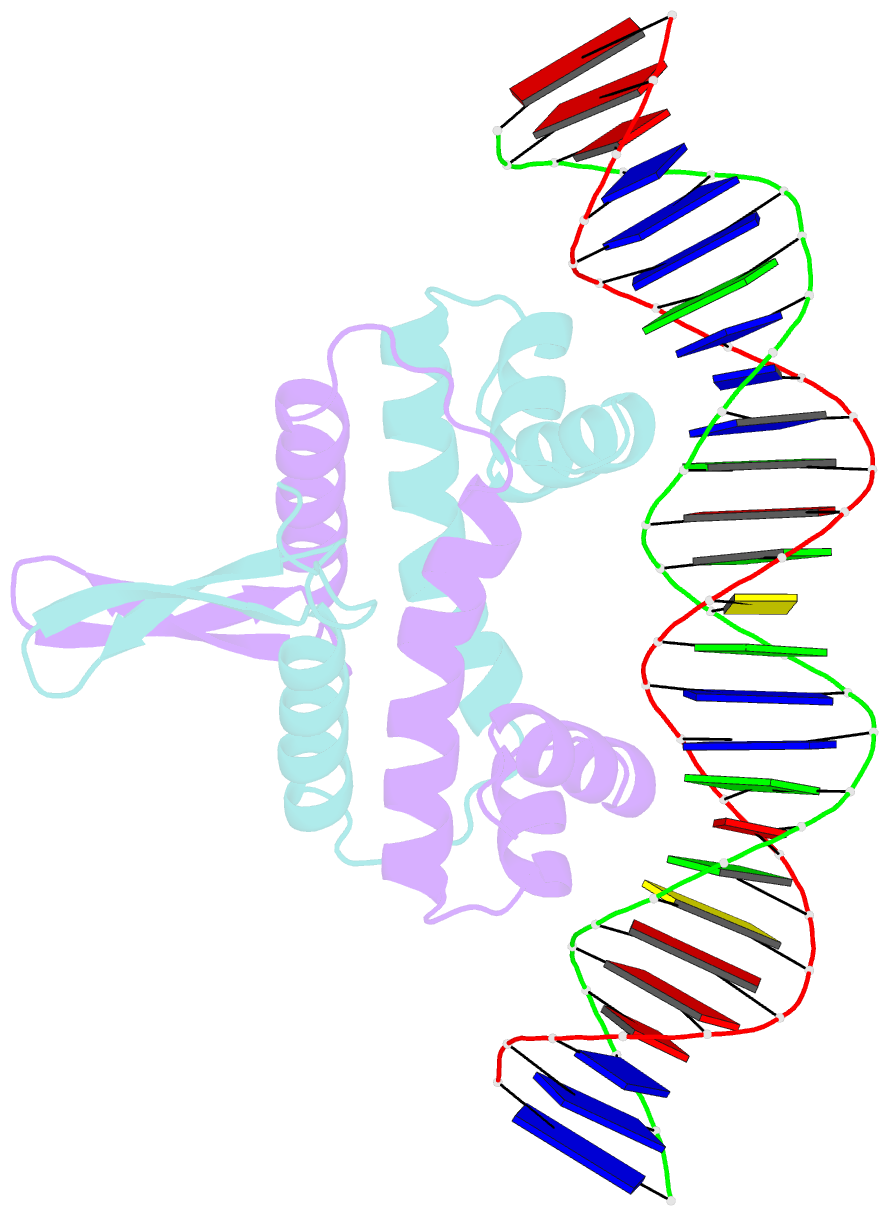
- Crystal structure of fis bound to 27 bp inosine substituted DNA f28-di (aaatttgtttgaicittgagcaaattt)
- Reference
- Hancock SP, Ghane T, Cascio D, Rohs R, Di Felice R, Johnson RC (2013): "Control of DNA minor groove width and Fis protein binding by the purine 2-amino group." Nucleic Acids Res., 41, 6750-6760. doi: 10.1093/nar/gkt357.
- Abstract
- The width of the DNA minor groove varies with sequence and can be a major determinant of DNA shape recognition by proteins. For example, the minor groove within the center of the Fis-DNA complex narrows to about half the mean minor groove width of canonical B-form DNA to fit onto the protein surface. G/C base pairs within this segment, which is not contacted by the Fis protein, reduce binding affinities up to 2000-fold over A/T-rich sequences. We show here through multiple X-ray structures and binding properties of Fis-DNA complexes containing base analogs that the 2-amino group on guanine is the primary molecular determinant controlling minor groove widths. Molecular dynamics simulations of free-DNA targets with canonical and modified bases further demonstrate that sequence-dependent narrowing of minor groove widths is modulated almost entirely by the presence of purine 2-amino groups. We also provide evidence that protein-mediated phosphate neutralization facilitates minor groove compression and is particularly important for binding to non-optimally shaped DNA duplexes.