Summary information and primary citation
- PDB-id
- 4itq; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation, structural protein-DNA
- Method
- X-ray (2.7 Å)
- Summary
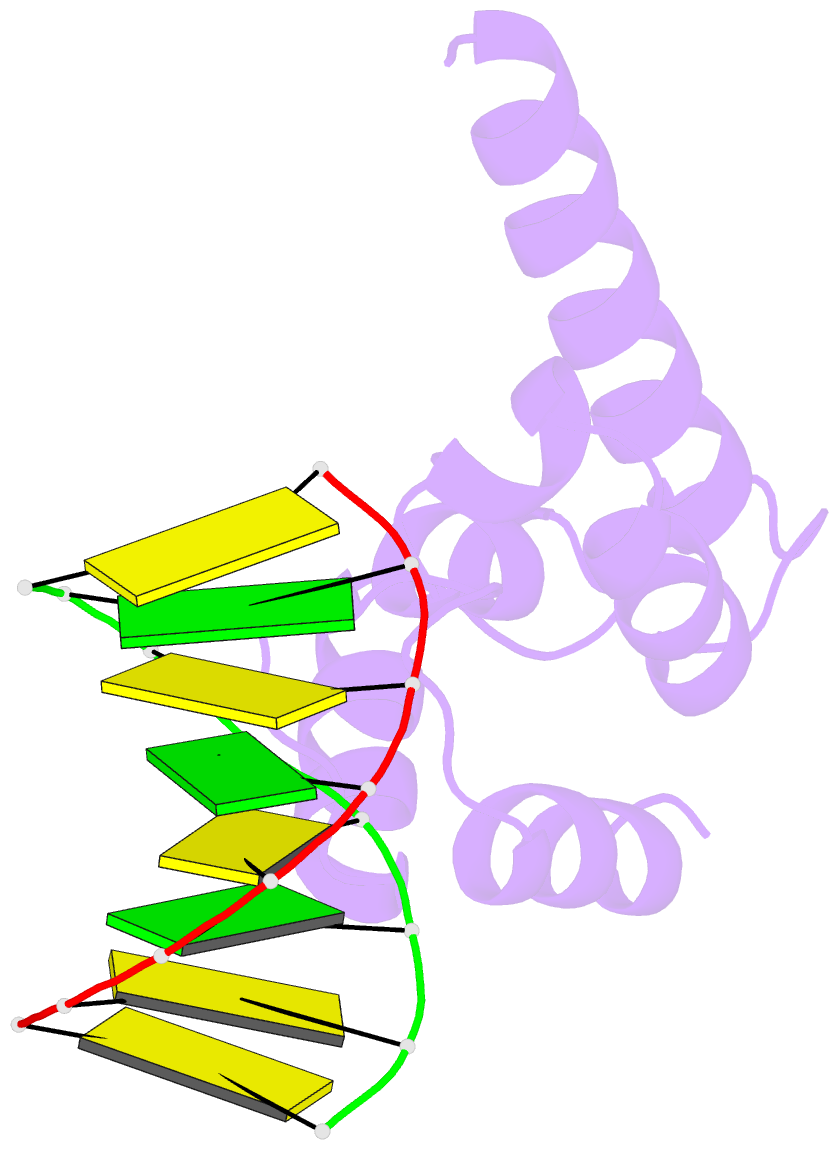
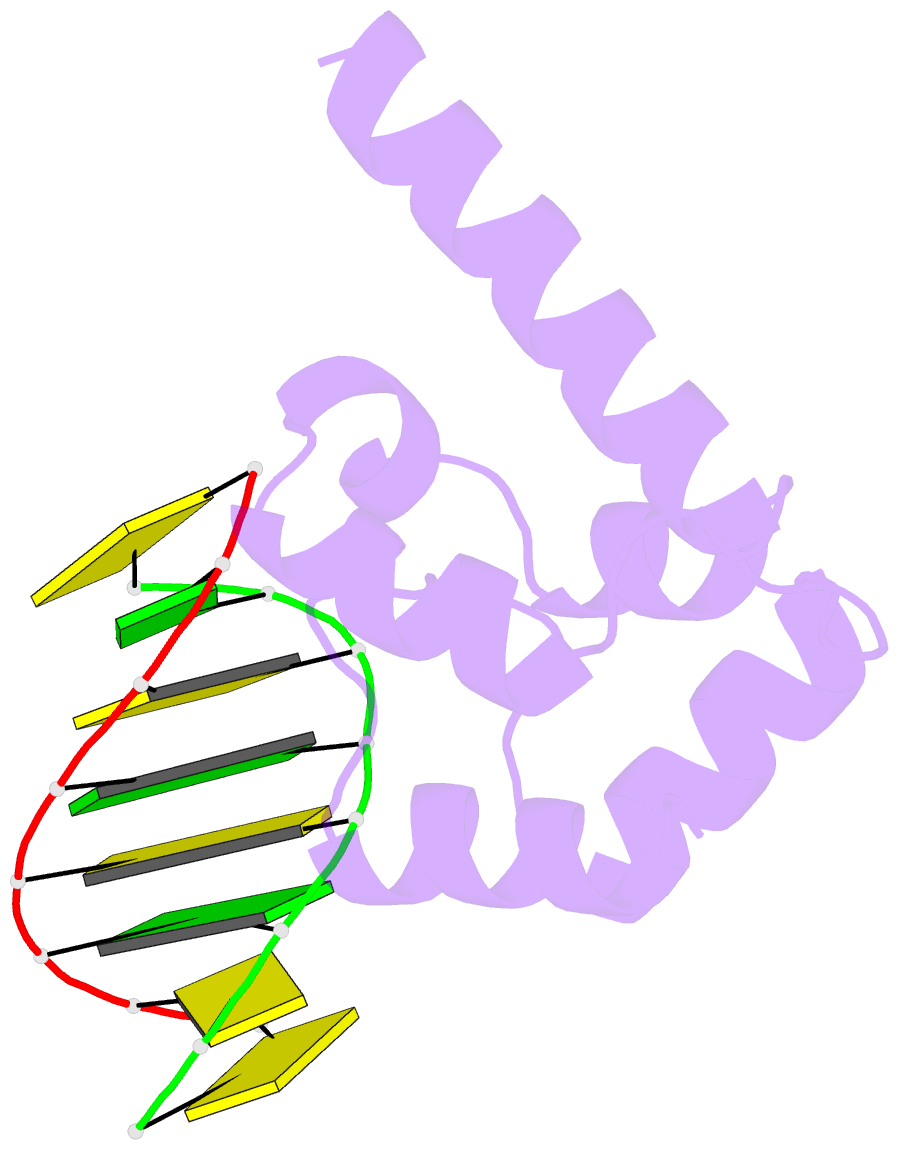
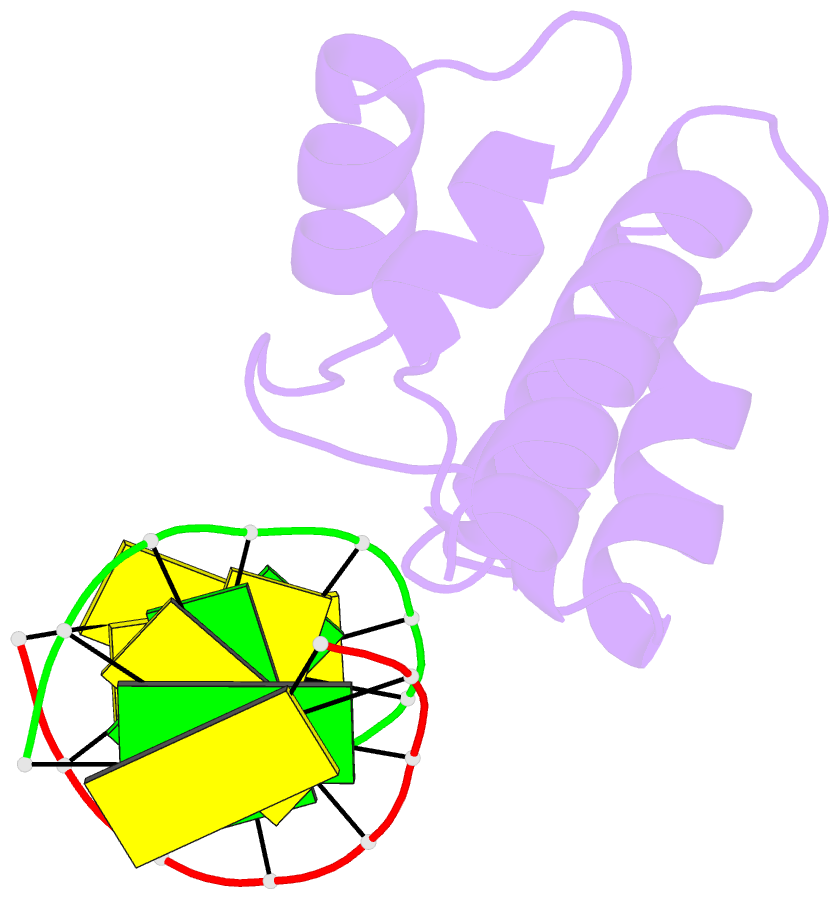
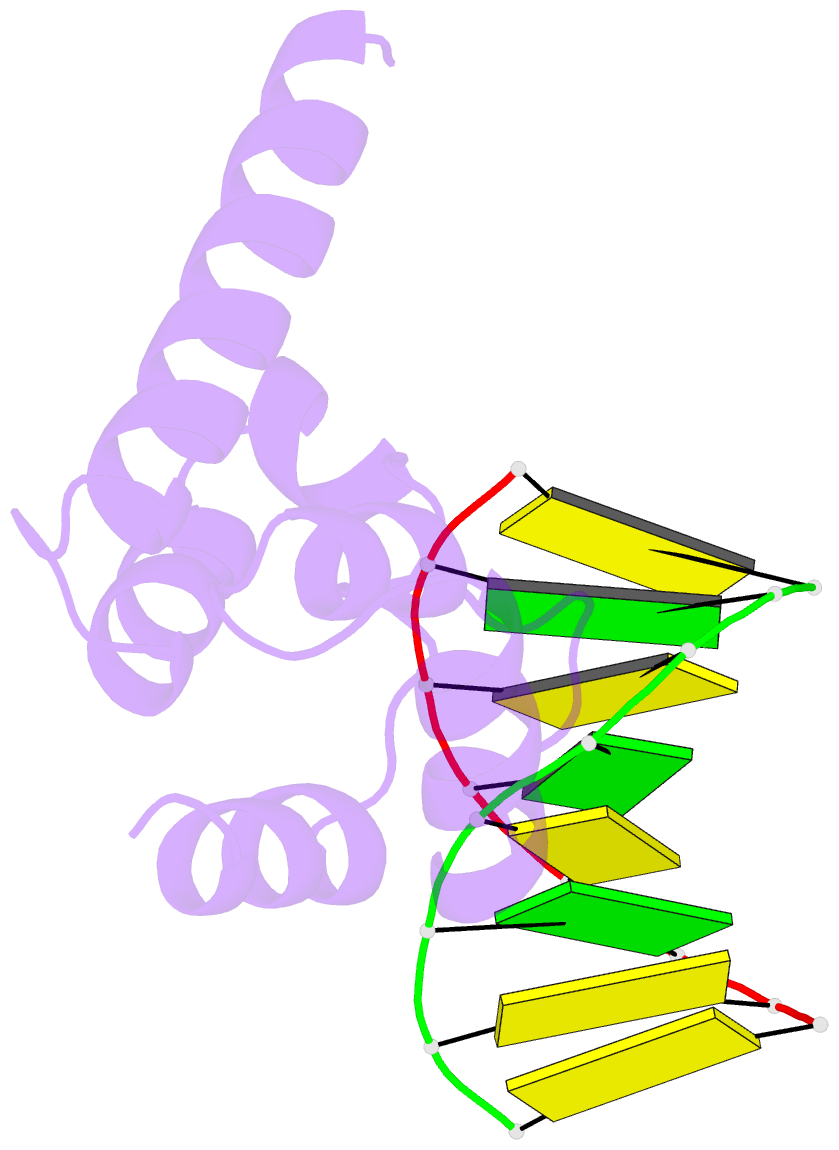
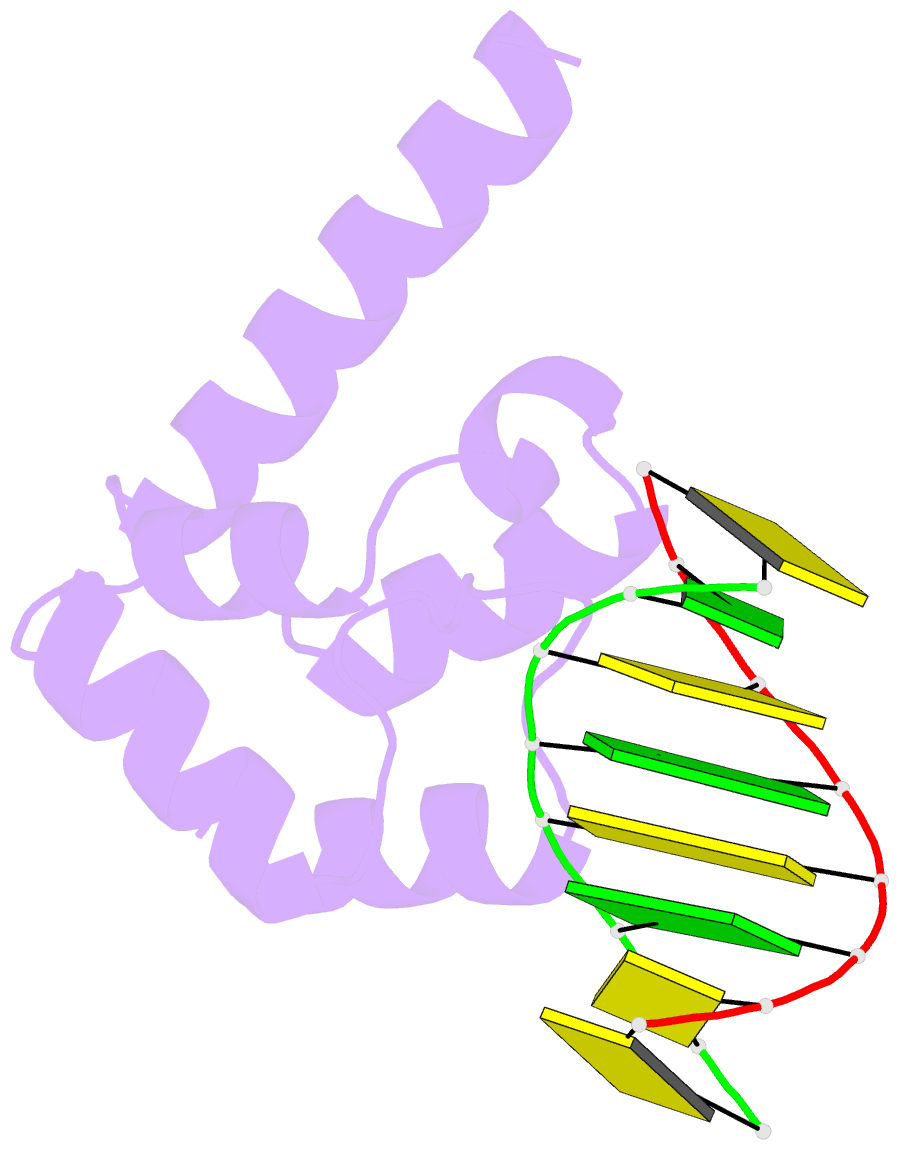
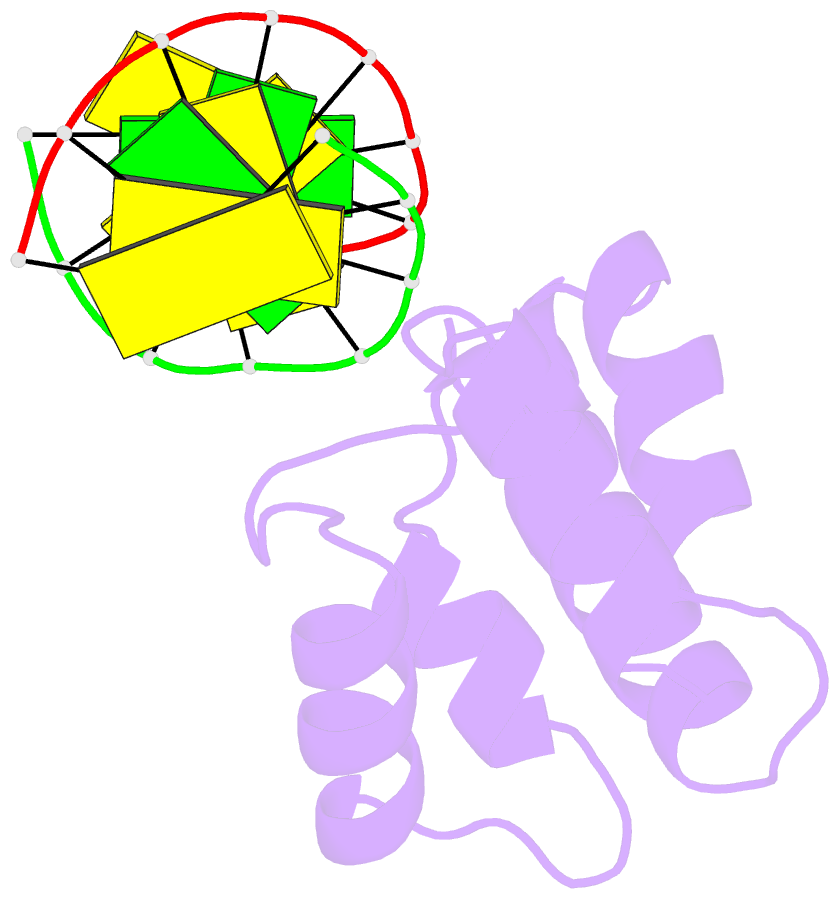
- Crystal structure of hypothetical protein sco1480 bound to DNA
- Reference
- Swiercz JP, Nanji T, Gloyd M, Guarne A, Elliot MA (2013): "A novel nucleoid-associated protein specific to the actinobacteria." Nucleic Acids Res., 41, 4171-4184. doi: 10.1093/nar/gkt095.
- Abstract
- Effective chromosome organization is central to the functioning of any cell. In bacteria, this organization is achieved through the concerted activity of multiple nucleoid-associated proteins. These proteins are not, however, universally conserved, and different groups of bacteria have distinct subsets that contribute to chromosome architecture. Here, we describe the characterization of a novel actinobacterial-specific protein in Streptomyces coelicolor. We show that sIHF (SCO1480) associates with the nucleoid and makes important contributions to chromosome condensation and chromosome segregation during Streptomyces sporulation. It also affects antibiotic production, suggesting an additional role in gene regulation. In vitro, sIHF binds DNA in a length-dependent but sequence-independent manner, without any obvious structural preferences. It does, however, impact the activity of topoisomerase, significantly altering DNA topology. The sIHF-DNA co-crystal structure reveals sIHF to be composed of two domains: a long N-terminal helix and a C-terminal helix-two turns-helix domain with two separate DNA interaction sites, suggesting a potential role in bridging DNA molecules.