Summary information and primary citation
- PDB-id
- 4j01; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.246 Å)
- Summary
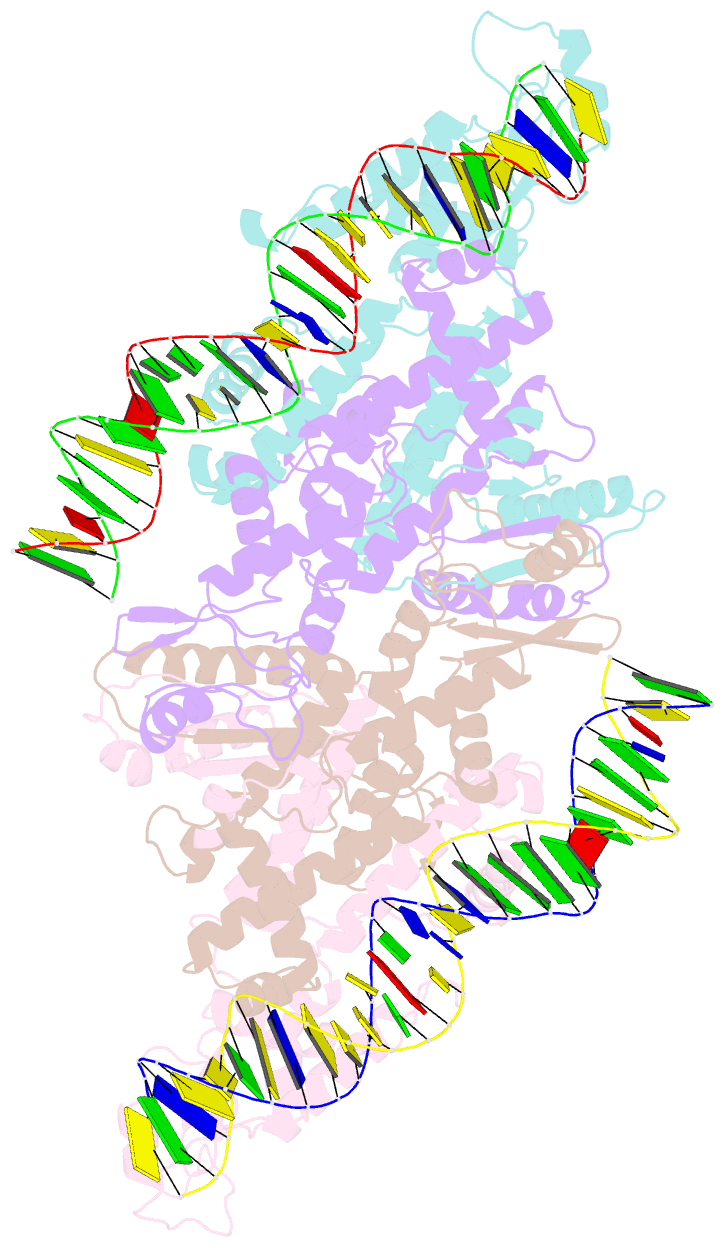
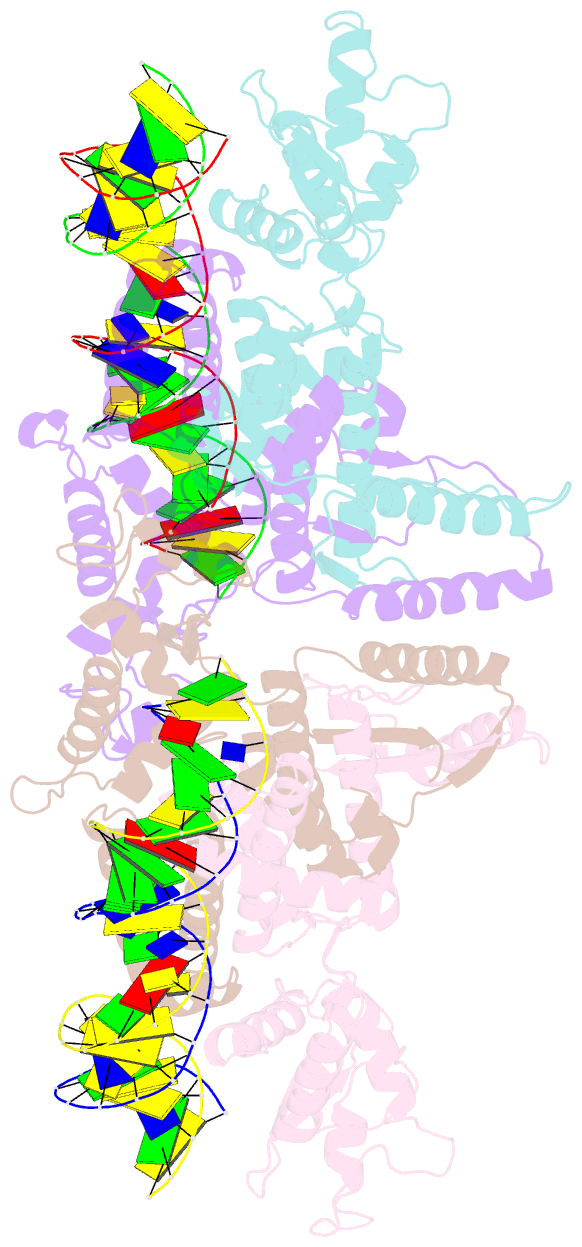
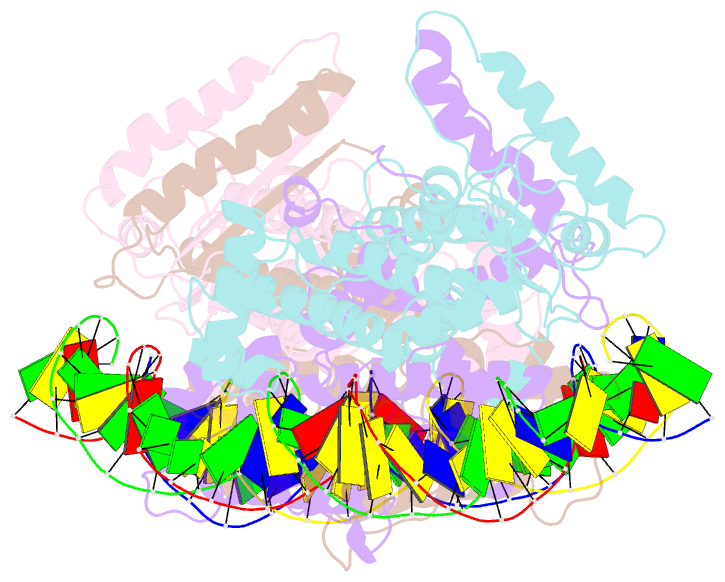
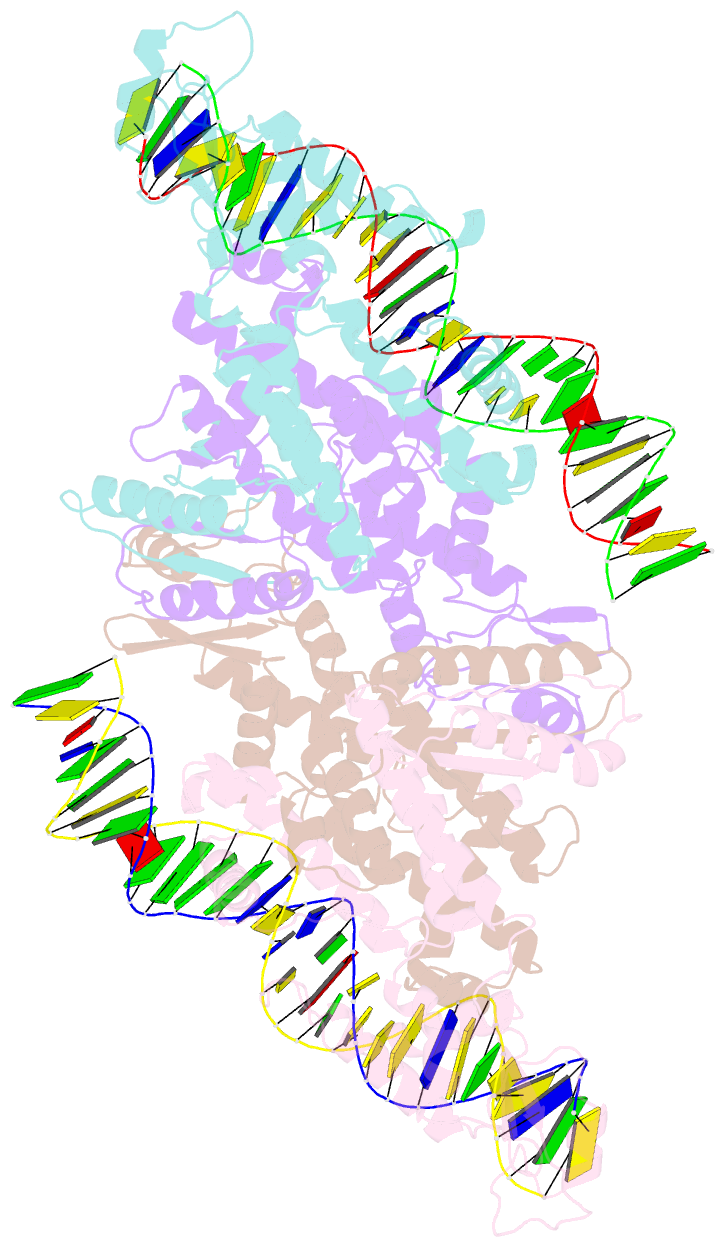
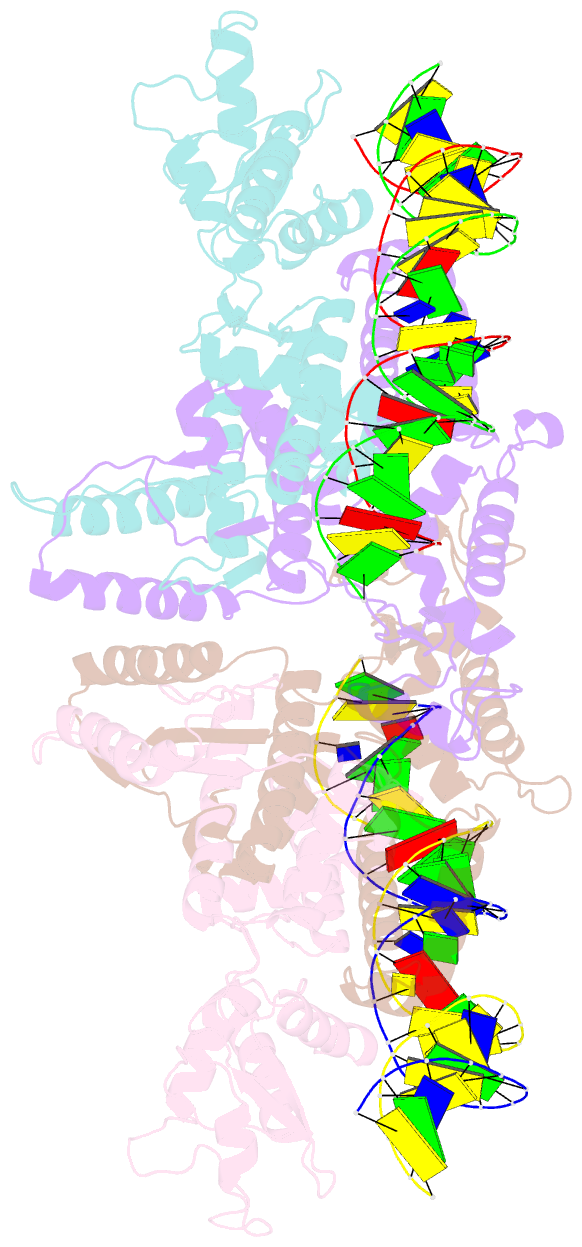
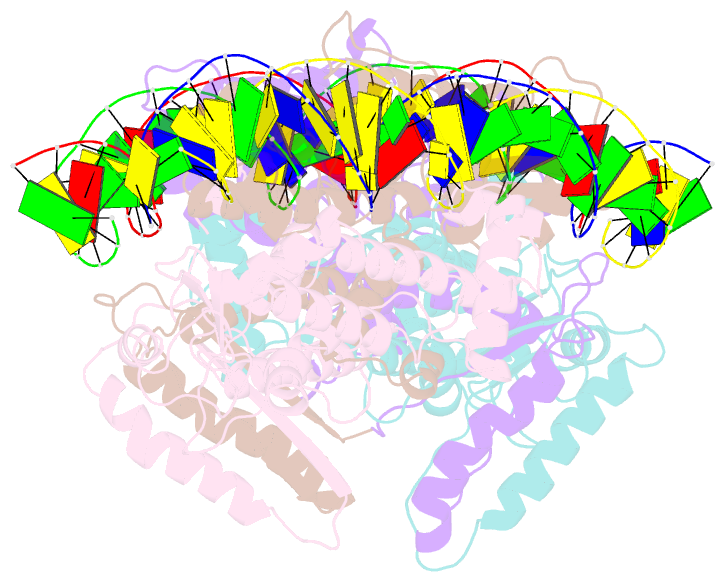
- Crystal structure of fischerella transcription factor hetr complexed with 29mer DNA target
- Reference
- Kim Y, Ye Z, Joachimiak G, Videau P, Young J, Hurd K, Callahan SM, Gornicki P, Zhao J, Haselkorn R, Joachimiak A (2013): "Structures of complexes comprised of Fischerella transcription factor HetR with Anabaena DNA targets." Proc.Natl.Acad.Sci.USA, 110, E1716-E1723. doi: 10.1073/pnas.1305971110.
- Abstract
- HetR is an essential regulator of heterocyst development in cyanobacteria. Many mutations in HetR render Anabaena incapable of nitrogen fixation. The protein binds to a DNA palindrome upstream of hetP and other genes. We have determined the crystal structures of HetR complexed with palindromic DNA targets, 21, 23, and 29 bp at 2.50-, 3.00-, and 3.25-Å resolution, respectively. The highest-resolution structure shows fine details of specific protein-DNA interactions. The lower-resolution structures with longer DNA duplexes have similar interaction patterns and show how the flap domains interact with DNA in a sequence nonspecific fashion. Fifteen of 15 protein-DNA contacts predicted on the basis of the structure were confirmed by single amino acid mutations that abolished binding in vitro and complementation in vivo. A striking feature of the structure is the association of glutamate 71 from each subunit of the HetR dimer with three successive cytosines in each arm of the palindromic target, a feature that is conserved among all known heterocyst-forming cyanobacteria sequenced to date.