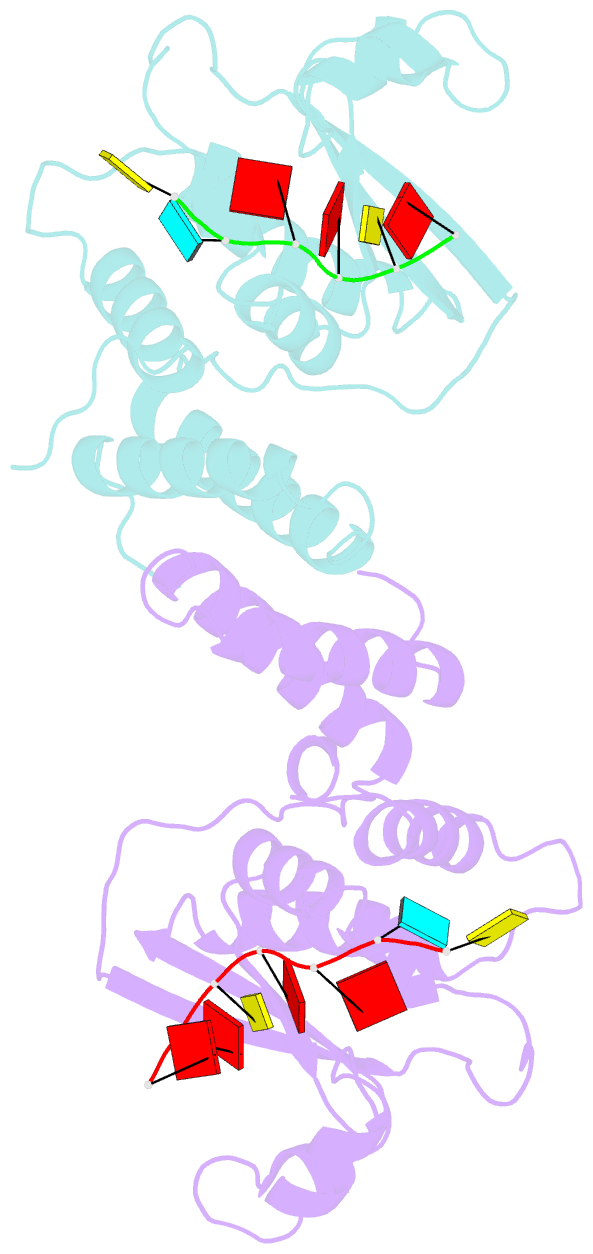
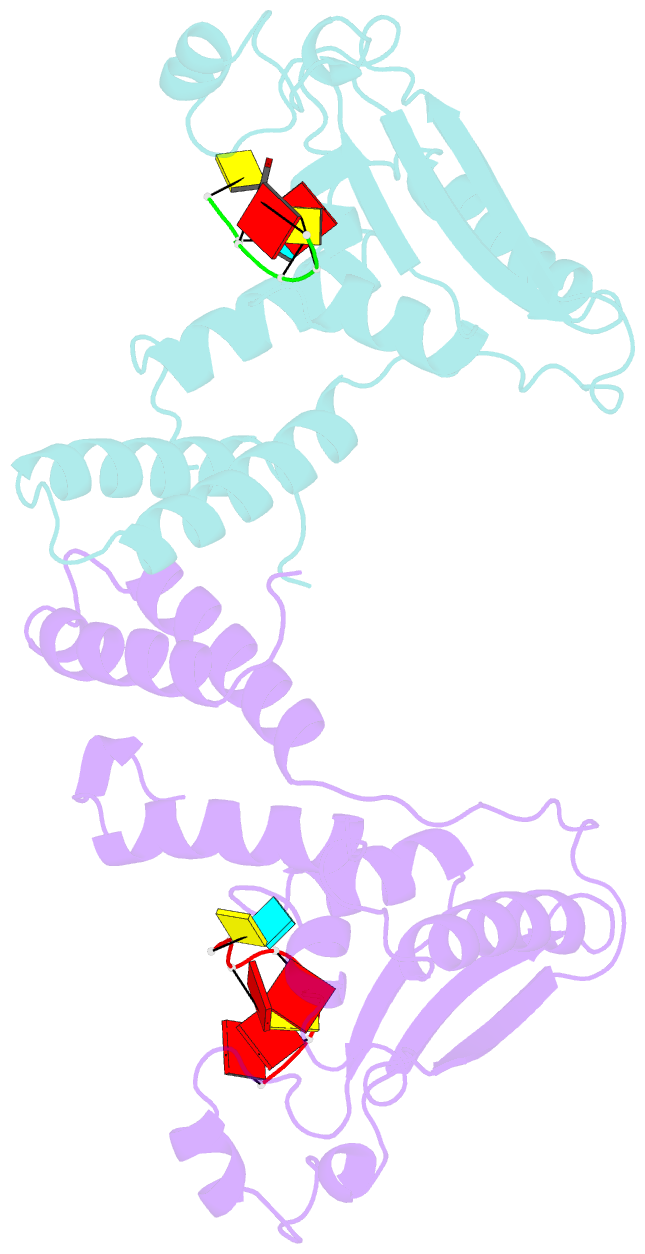
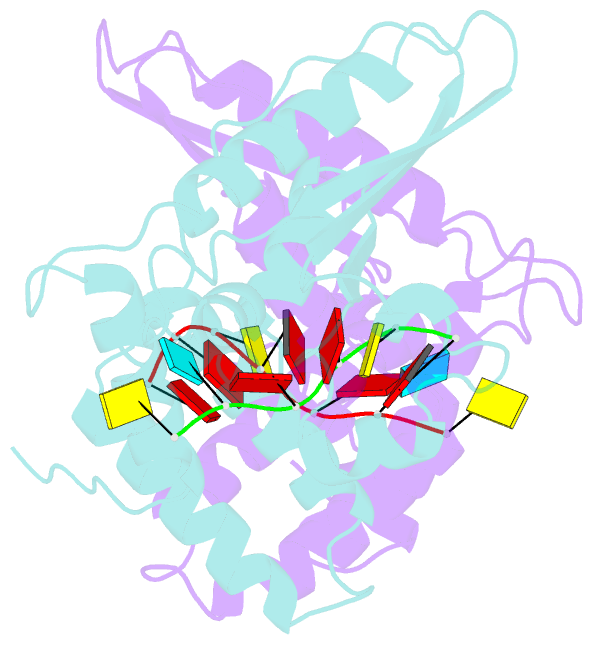
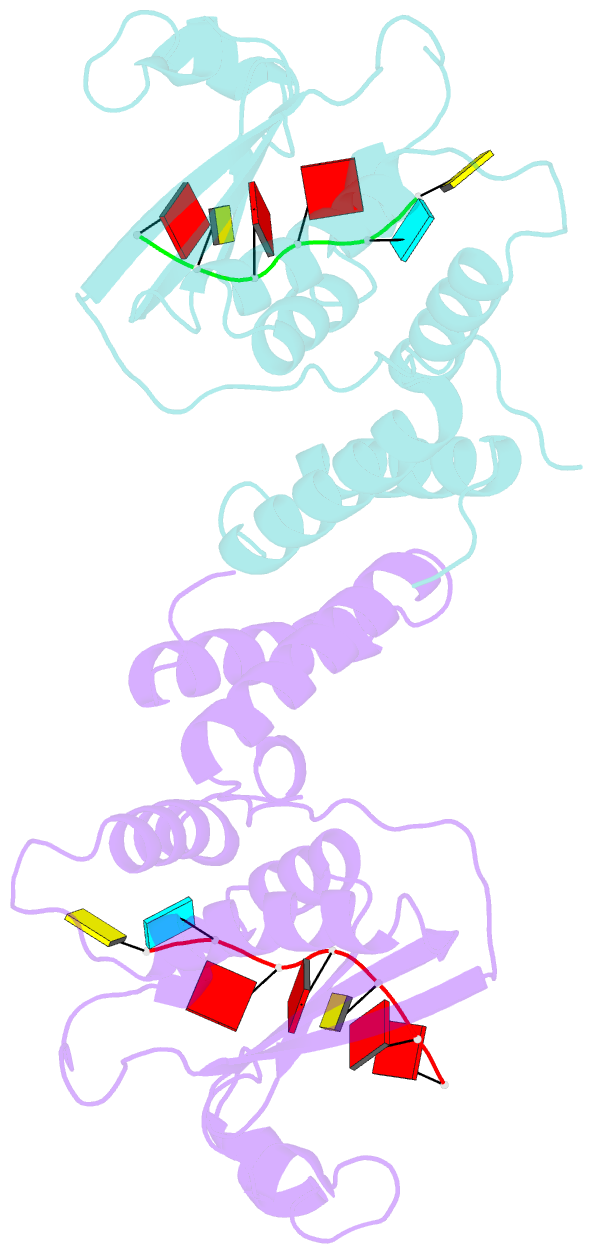
Summary information and primary citation
- PDB-id
-
4jvy;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (2.853 Å)
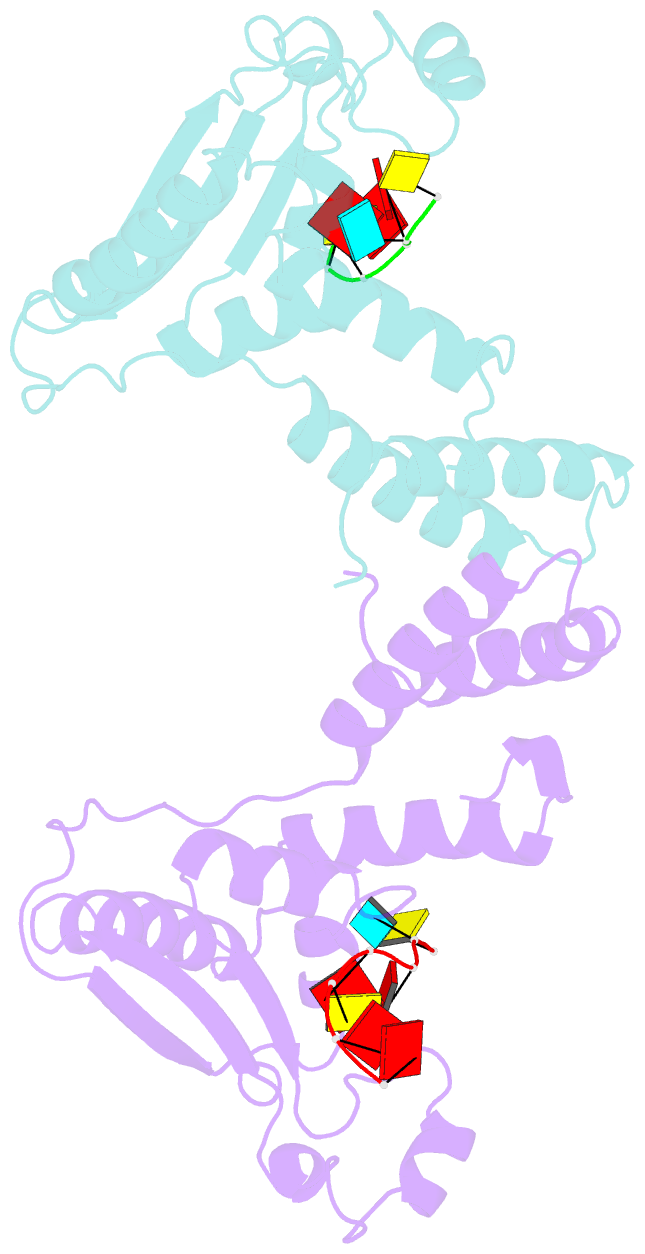
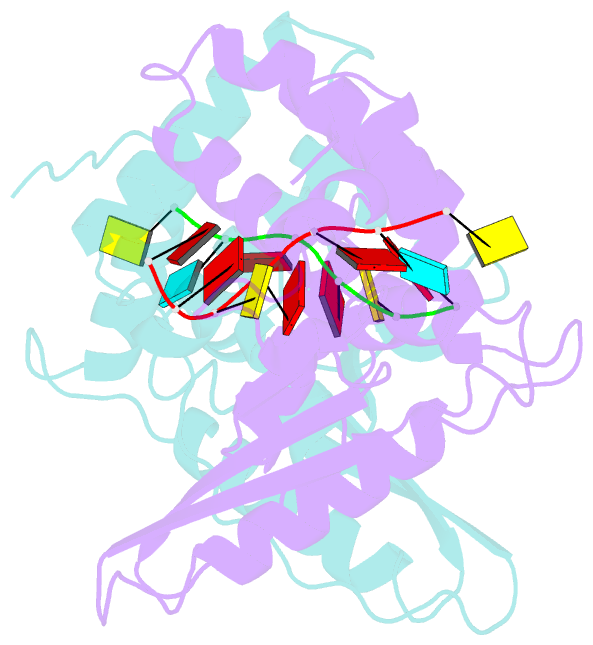
- Summary
- Structure of the star (signal transduction and
activation of RNA) domain of gld-1 bound to RNA
- Reference
-
Teplova M, Hafner M, Teplov D, Essig K, Tuschl T, Patel
DJ (2013): "Structure-function
studies of STAR family Quaking proteins bound to their in
vivo RNA target sites." Genes Dev.,
27, 928-940. doi: 10.1101/gad.216531.113.
- Abstract
- Mammalian Quaking (QKI) and its Caenorhabditis elegans
homolog, GLD-1 (defective in germ line development), are
evolutionarily conserved RNA-binding proteins, which
post-transcriptionally regulate target genes essential for
developmental processes and myelination. We present X-ray
structures of the STAR (signal transduction and activation
of RNA) domain, composed of Qua1, K homology (KH), and Qua2
motifs of QKI and GLD-1 bound to high-affinity in vivo RNA
targets containing YUAAY RNA recognition elements (RREs).
The KH and Qua2 motifs of the STAR domain synergize to
specifically interact with bases and sugar-phosphate
backbones of the bound RRE. Qua1-mediated homodimerization
generates a scaffold that enables concurrent recognition of
two RREs, thereby plausibly targeting tandem RREs present
in many QKI-targeted transcripts. Structure-guided
mutations reduced QKI RNA-binding affinity in vitro and in
vivo, and expression of QKI mutants in human embryonic
kidney cells (HEK293) significantly decreased the abundance
of QKI target mRNAs. Overall, our studies define principles
underlying RNA target selection by STAR homodimers and
provide insights into the post-transcriptional regulatory
function of mammalian QKI proteins.