Summary information and primary citation
- PDB-id
- 4kdp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.6 Å)
- Summary
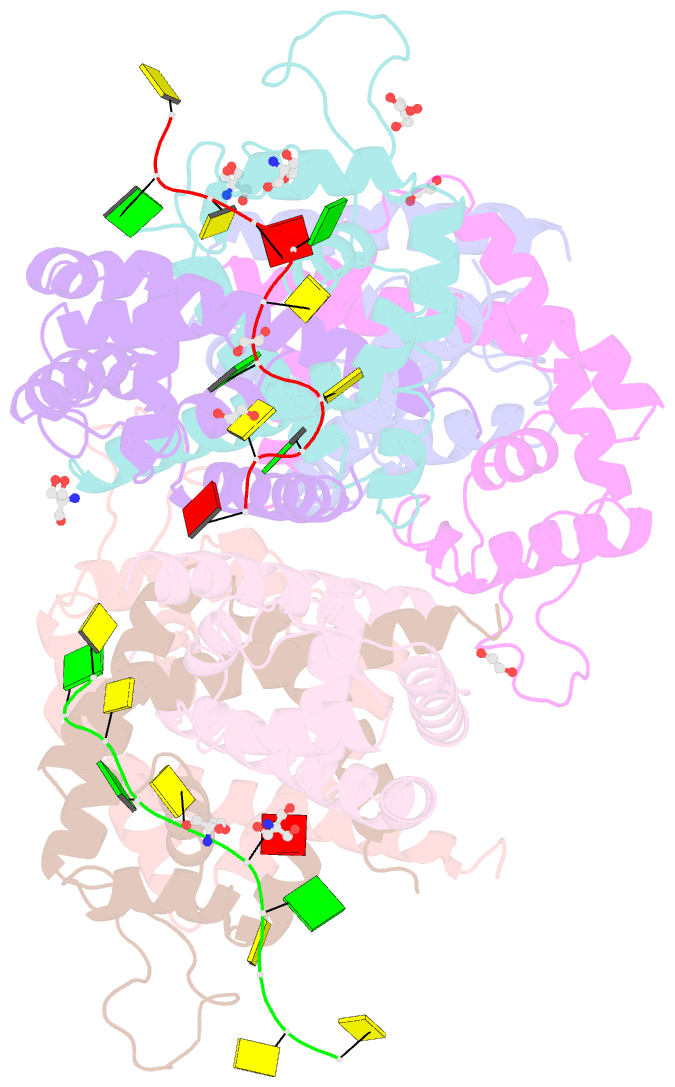
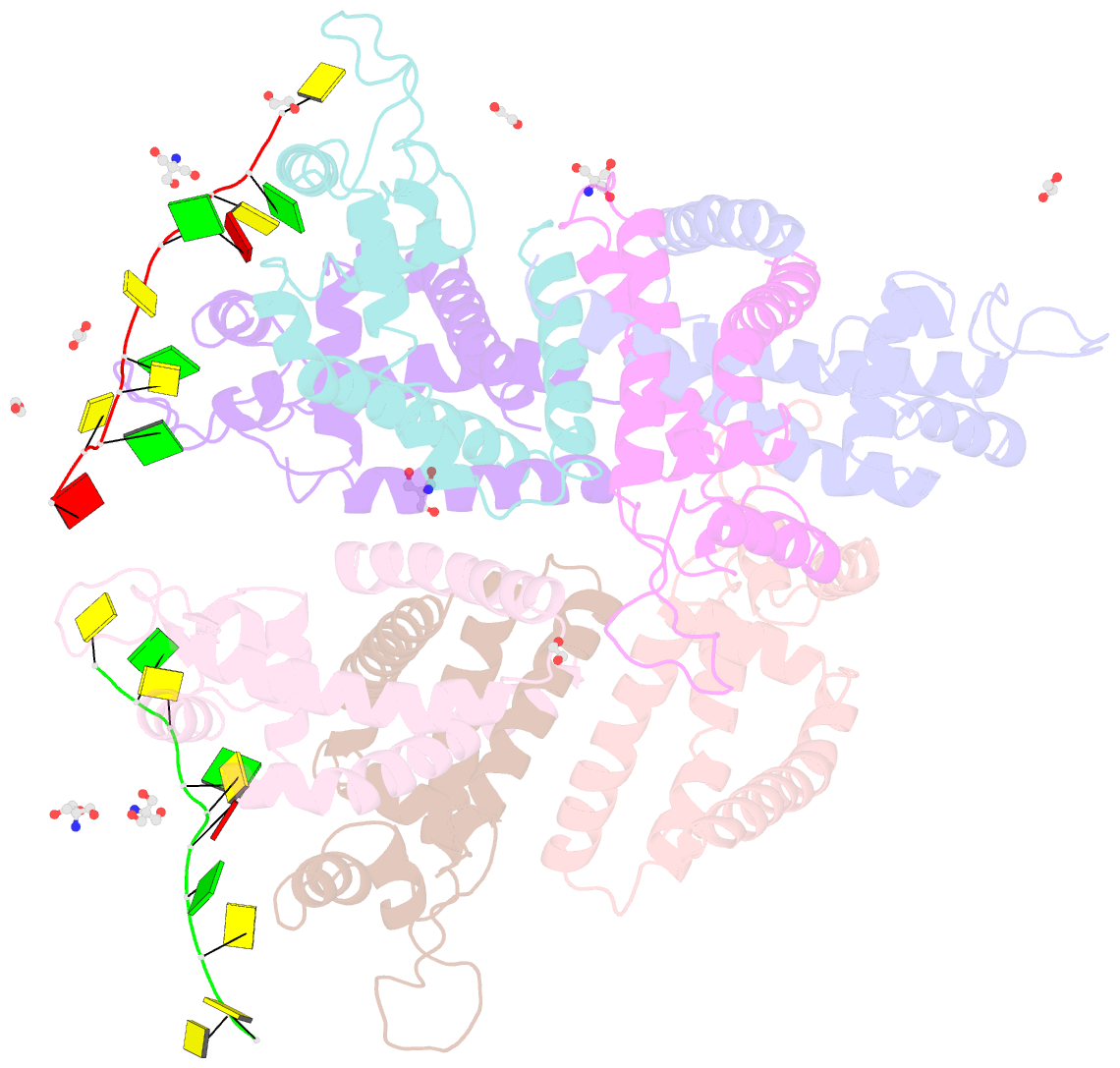
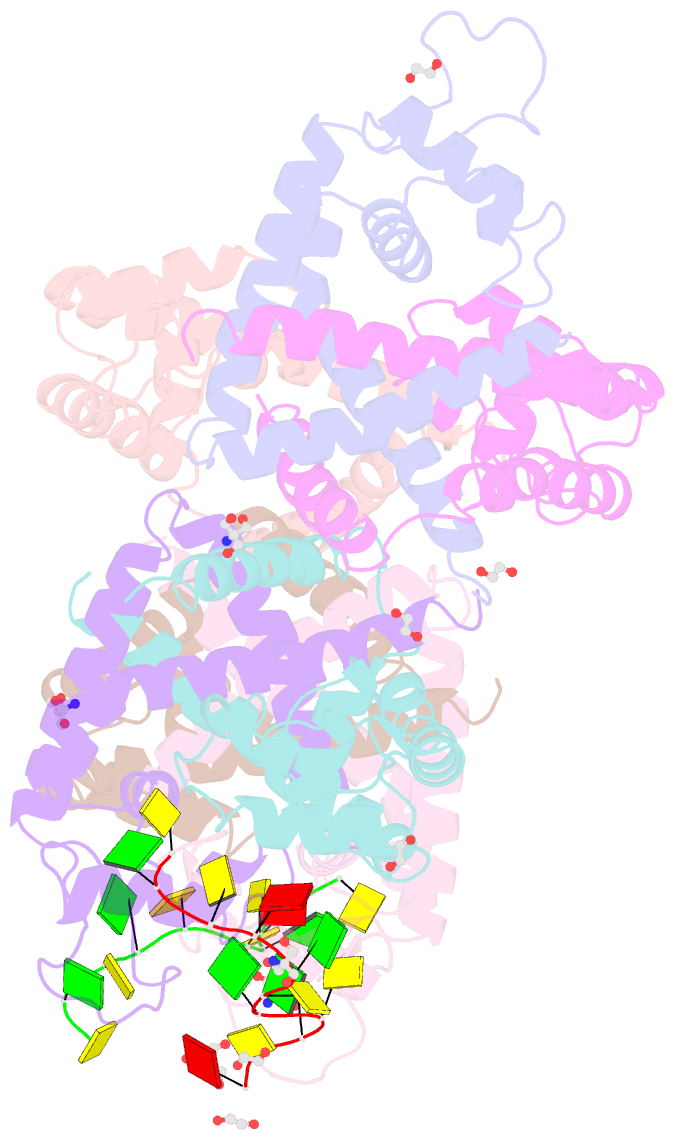
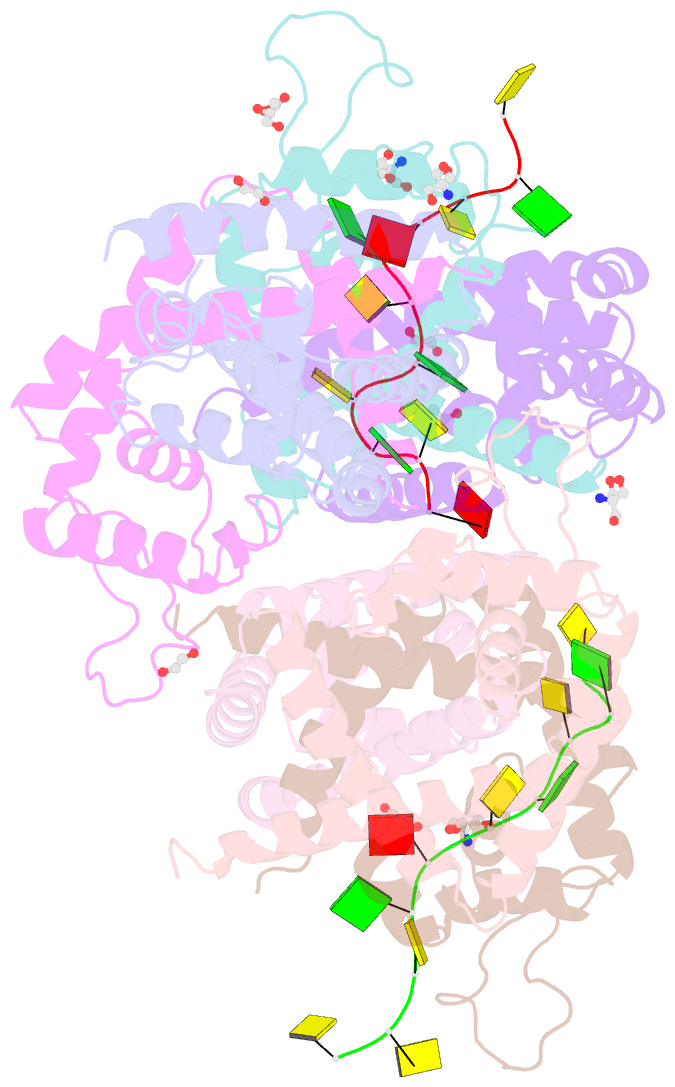
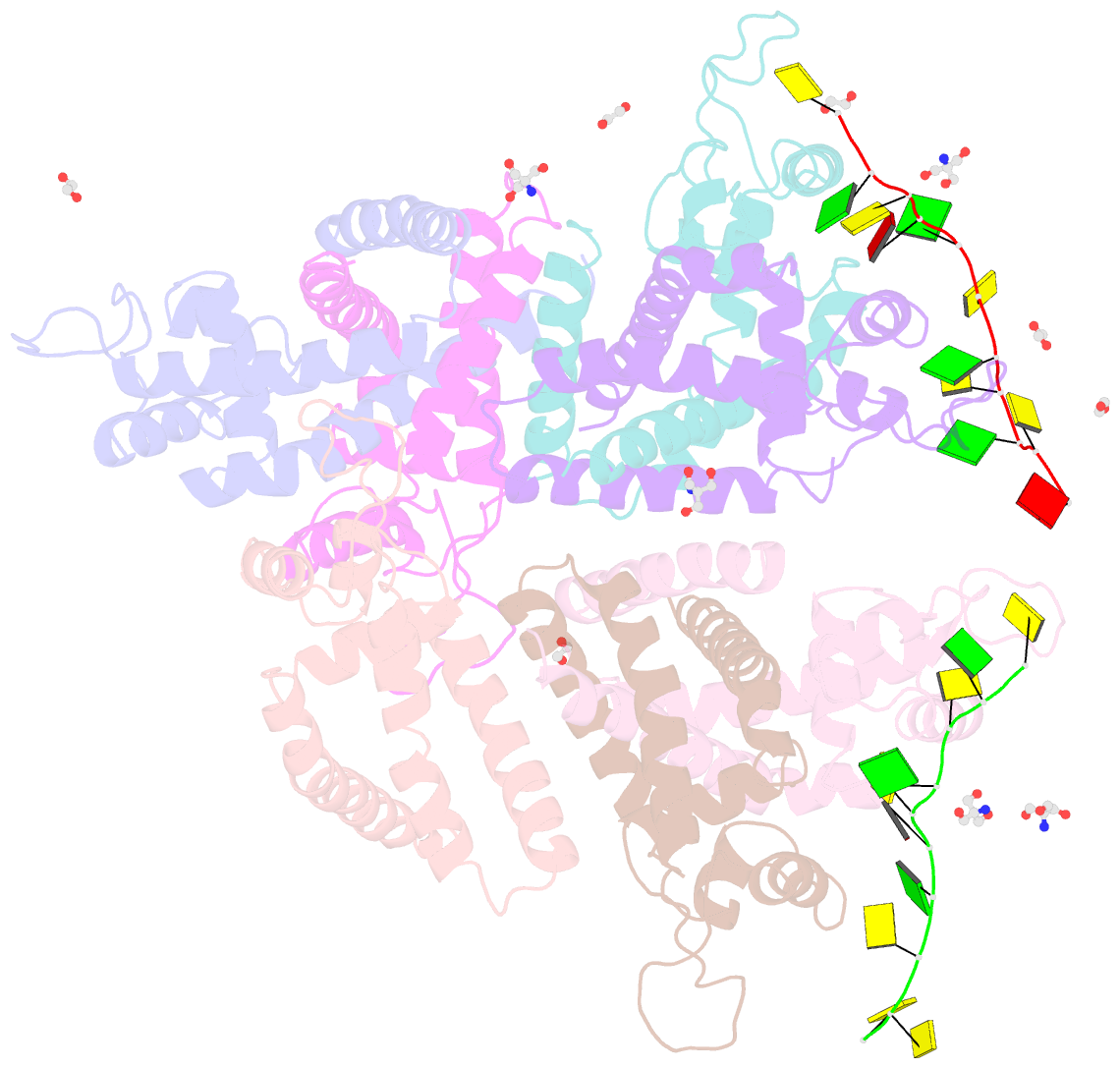
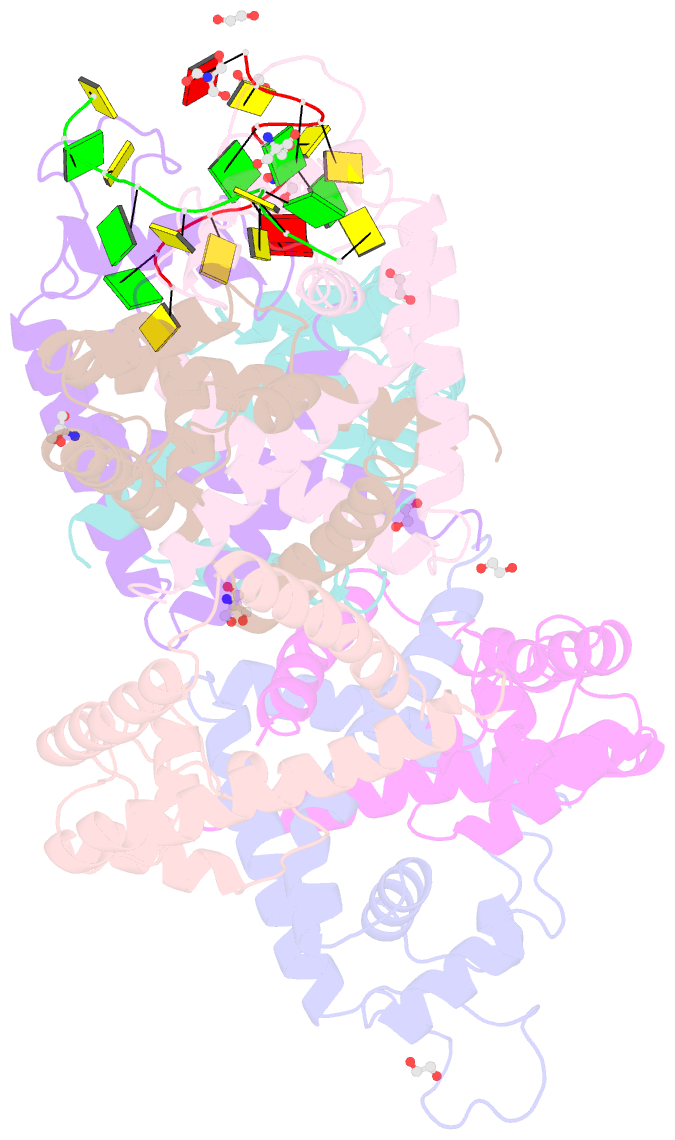
- Tcar-ssDNA complex crystal structure reveals the novel ssDNA binding mechanism of the marr family proteins
- Reference
- Chang YM, Ho CH, Chen CK, Maestre-Reyna M, Chang-Chien MW, Wang AH (2014): "TcaR-ssDNA complex crystal structure reveals new DNA binding mechanism of the MarR family proteins." Nucleic Acids Res., 42, 5314-5321. doi: 10.1093/nar/gku128.
- Abstract
- The teicoplanin-associated locus regulator (TcaR) regulates gene expression of proteins on the intercellular adhesion (ica) locus involved in staphylococci poly-N-acetylglucosamine biosynthesis. The absence of TcaR increases poly-N-acetylglucosamine production and promotes biofilm formation. Until recently, the mechanism of multiple antibiotic resistance regulator family protein members, such as TcaR, was restricted to binding double-stranded DNA. However, we recently found that TcaR strongly interacts with single-stranded DNA, which is a new role for this family of proteins. In this study, we report Staphylococcus epidermidis TcaR-single-stranded DNA complex structures. Our model suggests that TcaR and single-stranded DNA form a 61-symmetry polymer composed of TcaR dimers with single-stranded DNA that wraps outside the polymer and 12 nt per TcaR dimer. Single-stranded DNA binding to TcaR involves a large conformational change at the DNA binding lobe. Several point mutations involving the single-stranded DNA binding surface validate interactions between single-stranded DNA and TcaR. Our results extend the novel role of multiple antibiotic resistance regulator family proteins in staphylococci.