Summary information and primary citation
- PDB-id
- 4kud; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- structural protein-transcription-DNA
- Method
- X-ray (3.203 Å)
- Summary
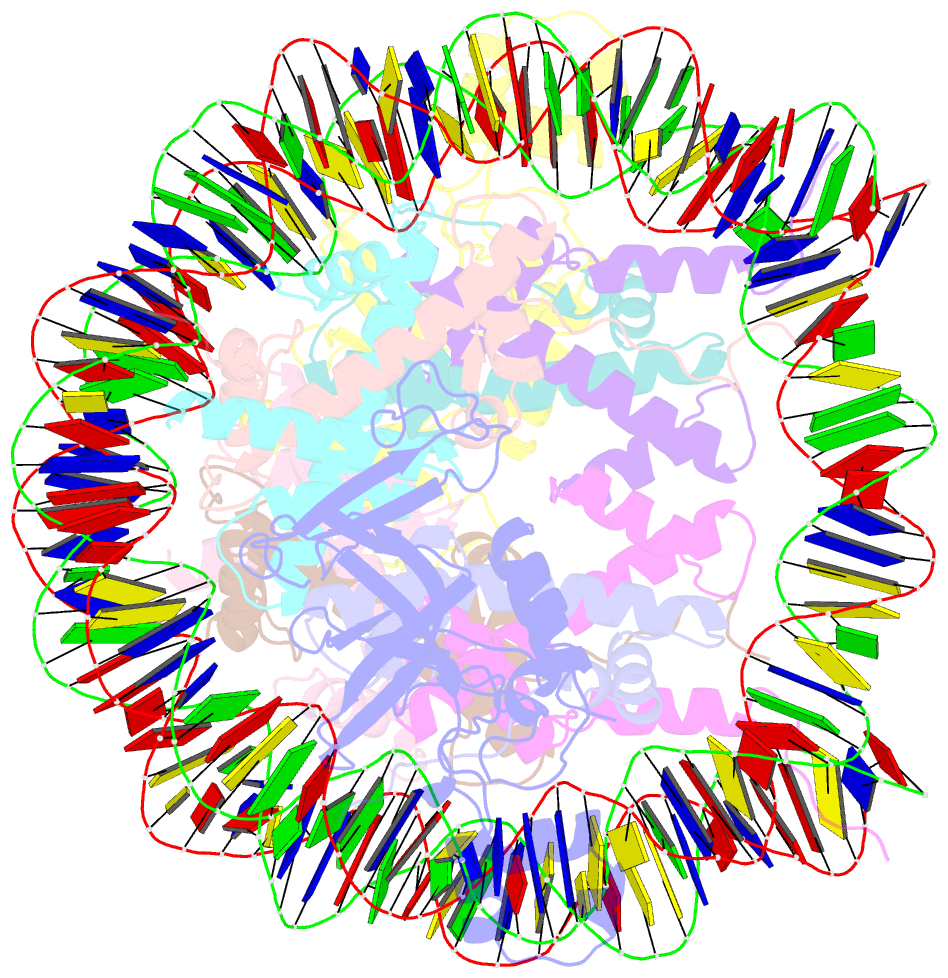
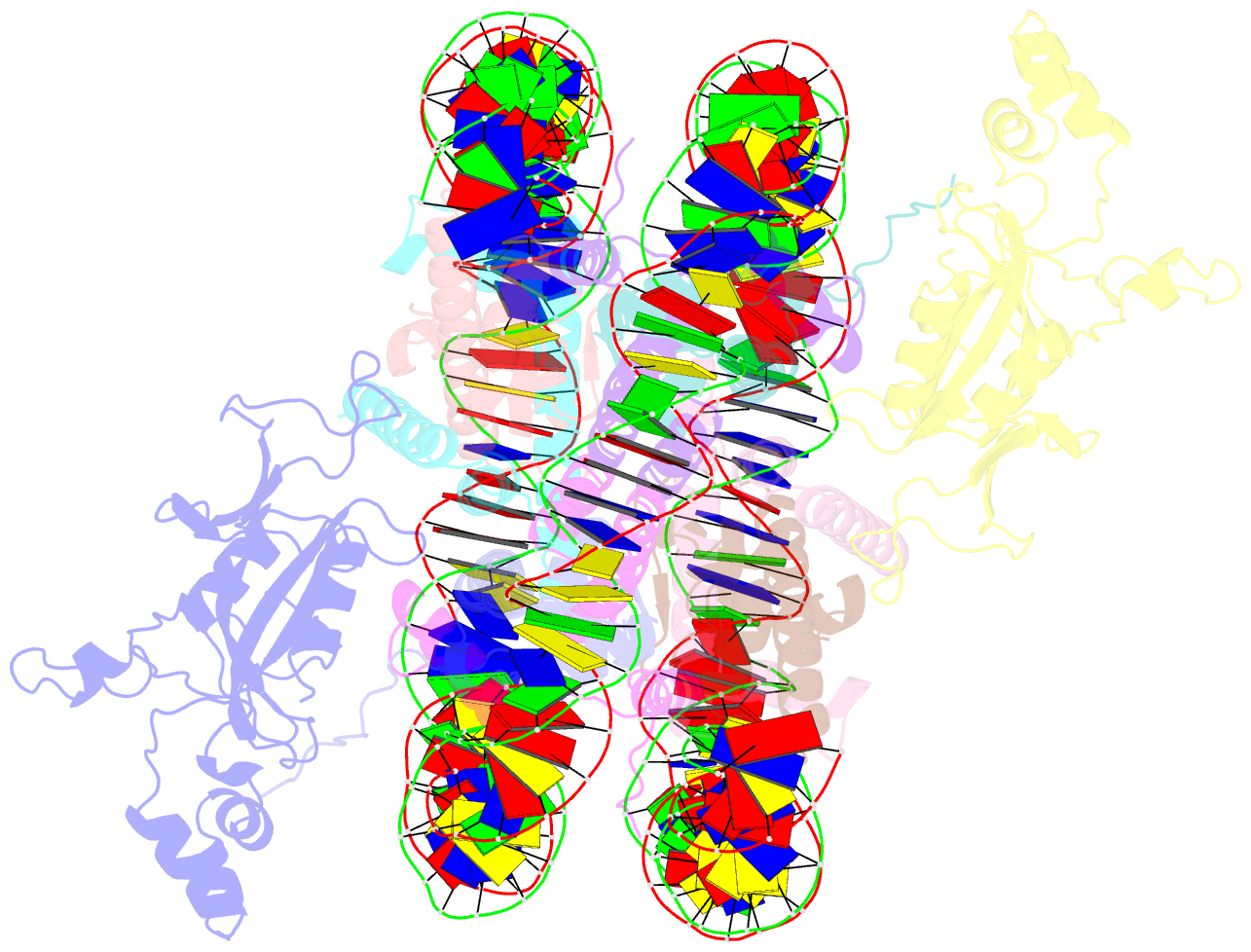
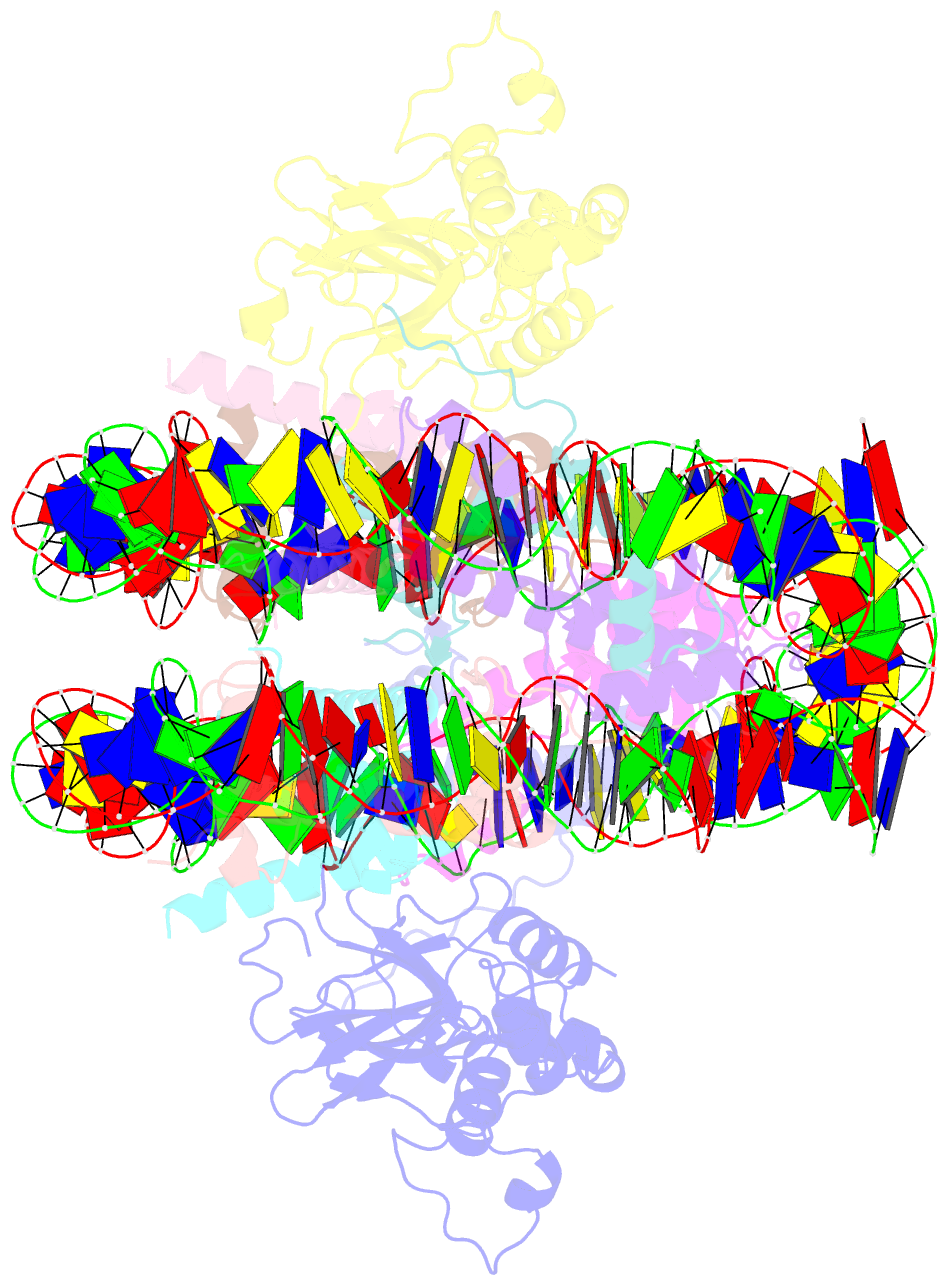
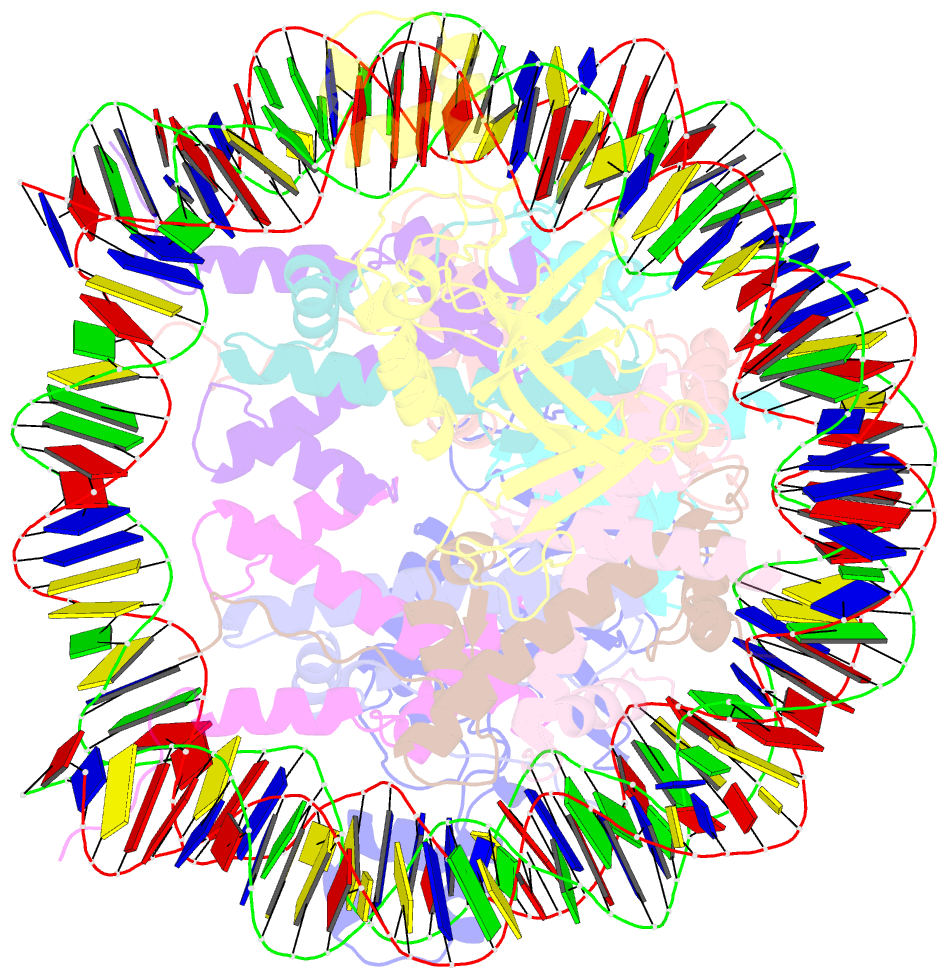
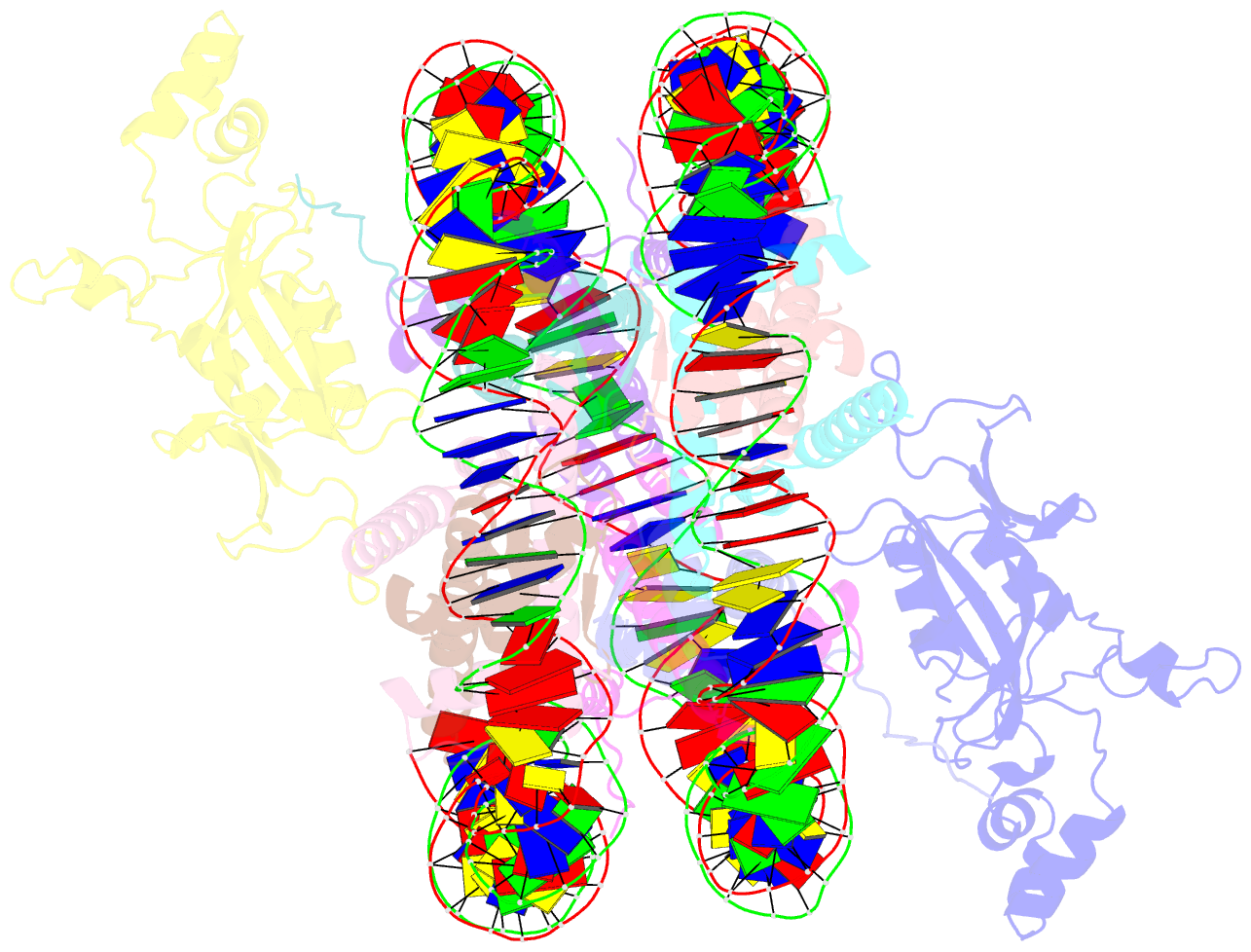
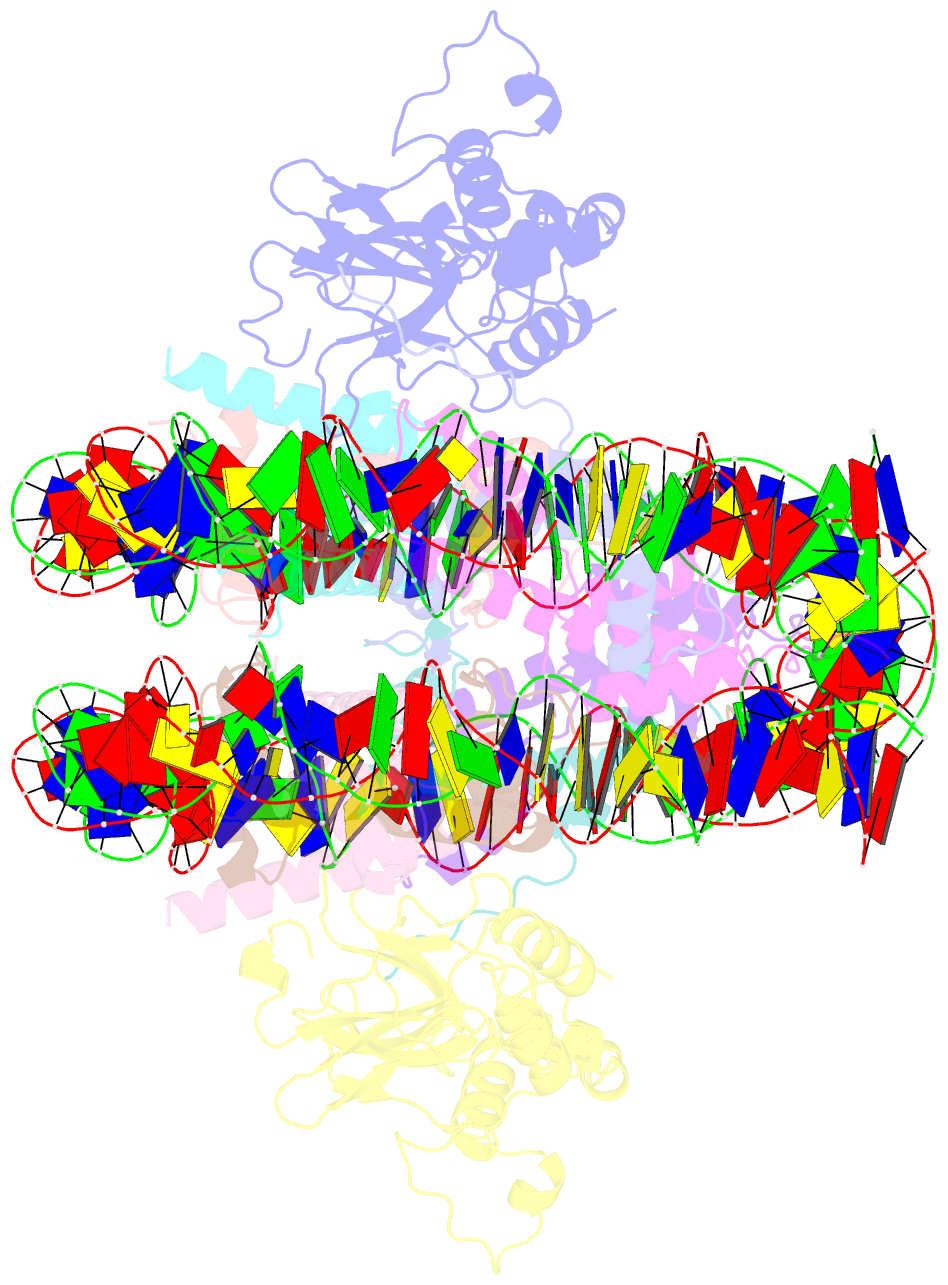
- Crystal structure of n-terminal acetylated sir3 bah domain d205n mutant in complex with yeast nucleosome core particle
- Reference
- Yang D, Fang Q, Wang M, Ren R, Wang H, He M, Sun Y, Yang N, Xu RM (2013): "N alpha-acetylated Sir3 stabilizes the conformation of a nucleosome-binding loop in the BAH domain." Nat.Struct.Mol.Biol., 20, 1116-1118. doi: 10.1038/nsmb.2637.
- Abstract
- In Saccharomyces cerevisiae, acetylation of the Sir3 N terminus is important for transcriptional silencing. This covalent modification promotes the binding of the Sir3 BAH domain to the nucleosome, but a mechanistic understanding of this phenomenon is lacking. By X-ray crystallography, we show here that the acetylated N terminus of Sir3 does not interact with the nucleosome directly. Instead, it stabilizes a nucleosome-binding loop in the BAH domain.