Summary information and primary citation
- PDB-id
- 4ld0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.75 Å)
- Summary
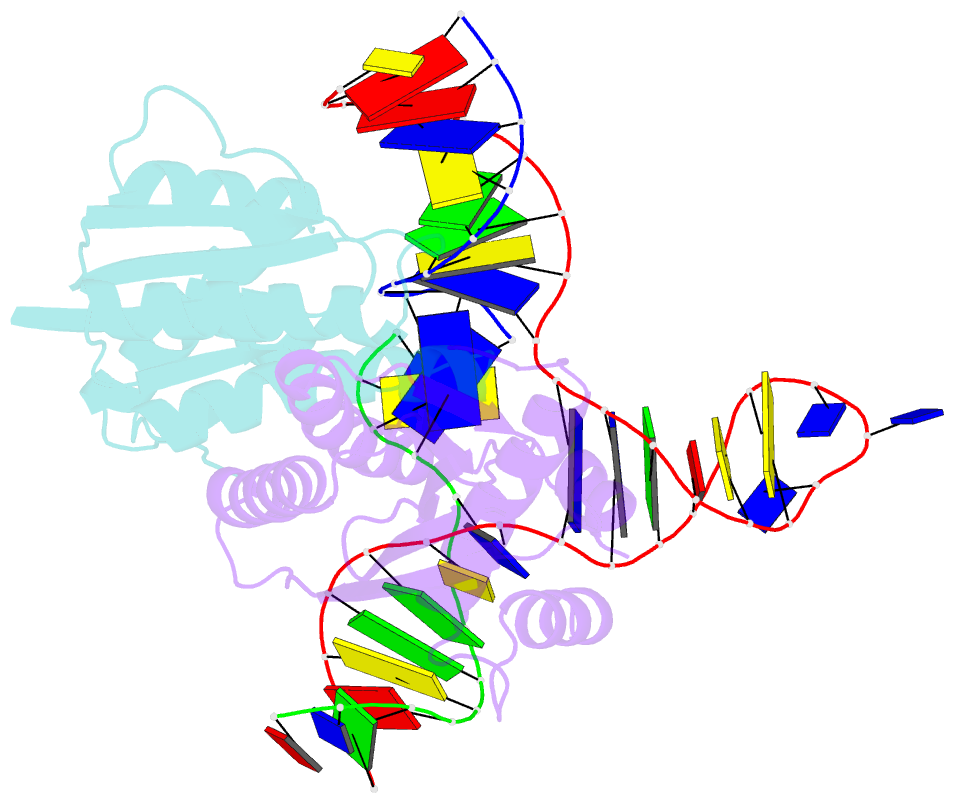
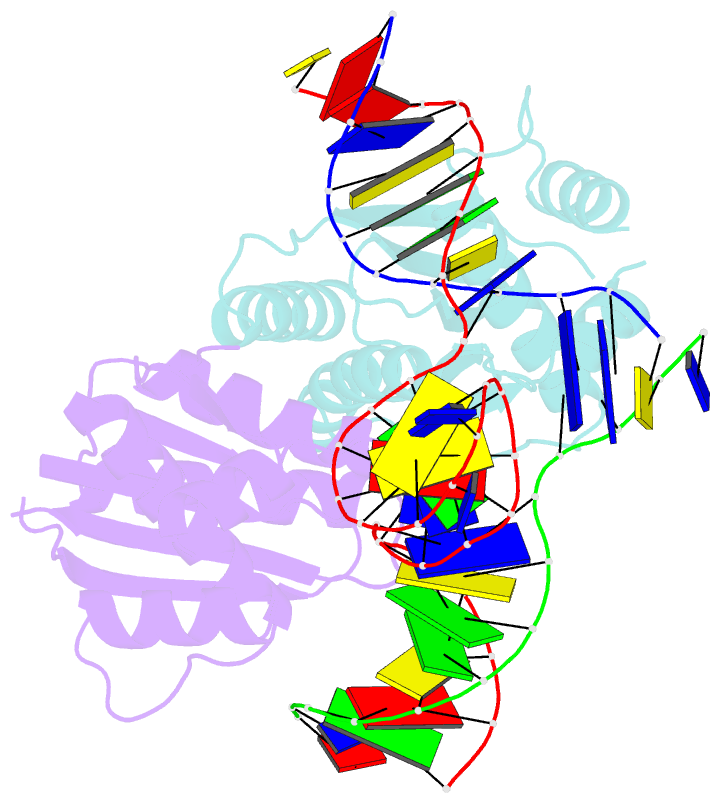
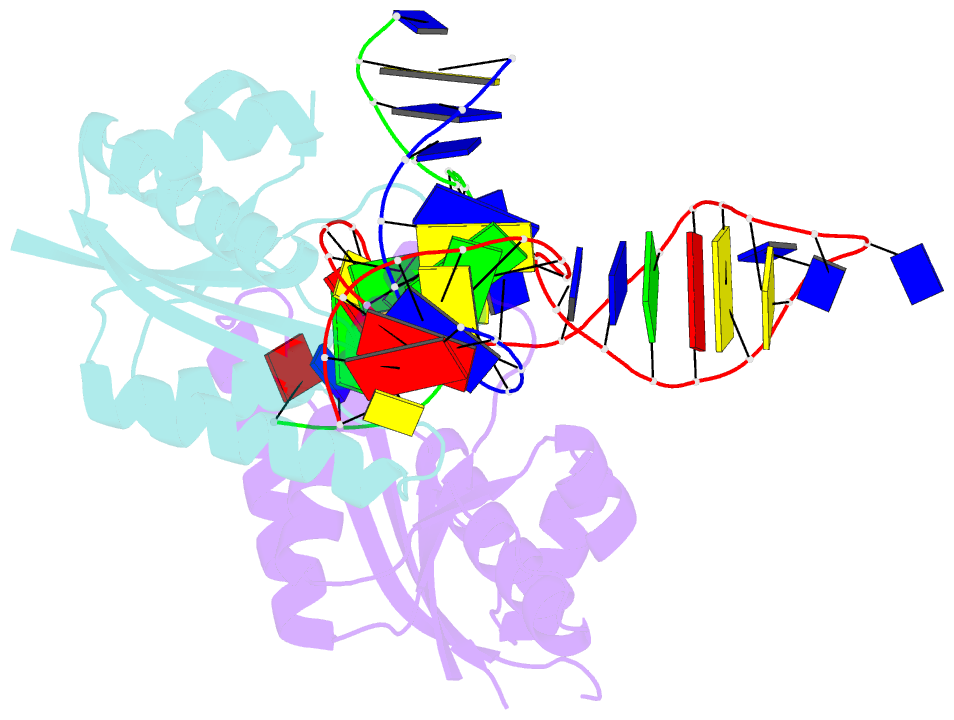
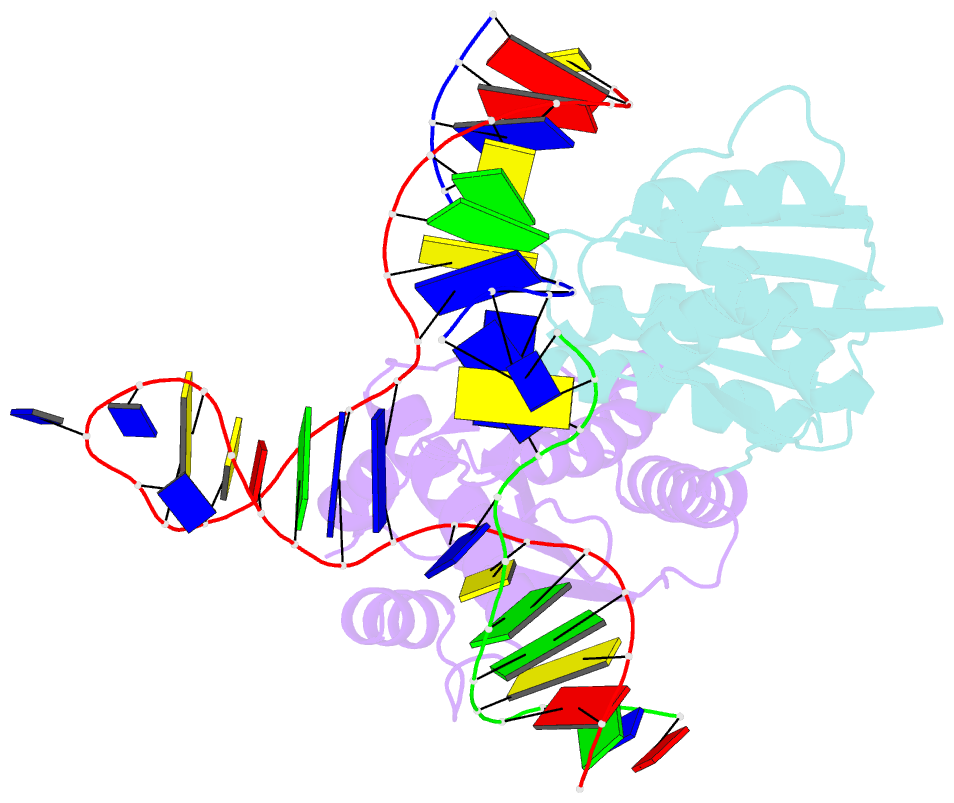
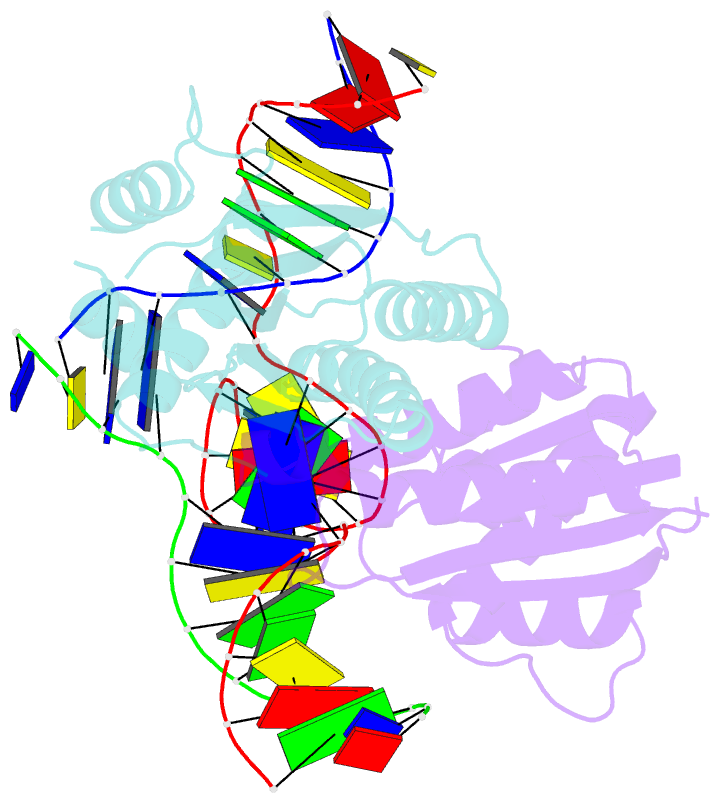
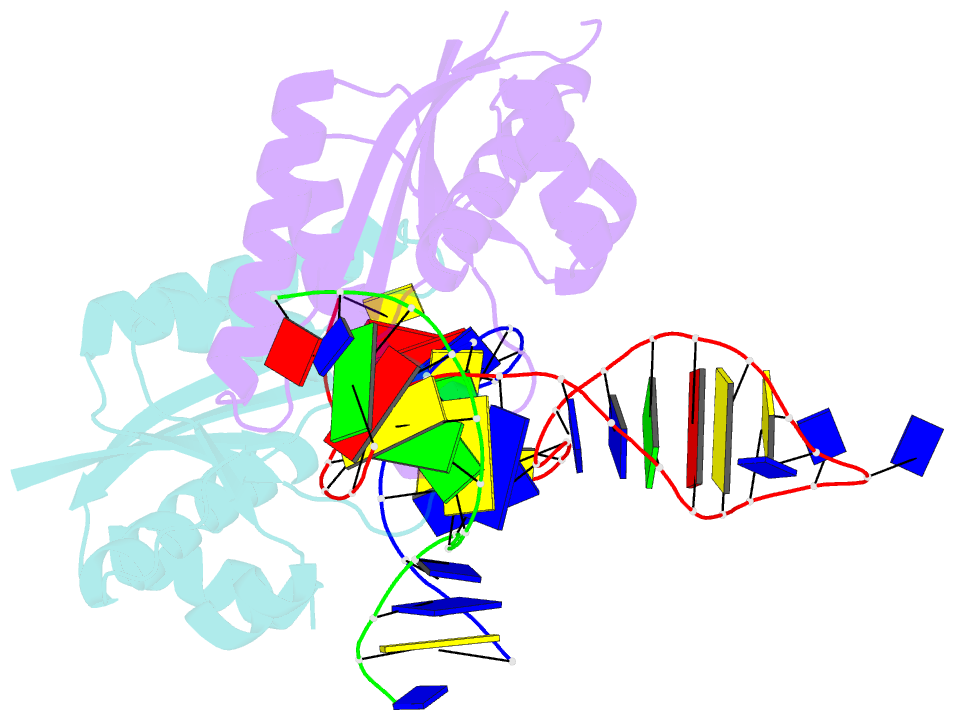
- T. thermophilus ruvc in complex with holliday junction substrate
- Reference
- Gorecka KM, Komorowska W, Nowotny M (2013): "Crystal structure of RuvC resolvase in complex with Holliday junction substrate." Nucleic Acids Res., 41, 9945-9955. doi: 10.1093/nar/gkt769.
- Abstract
- The key intermediate in genetic recombination is the Holliday junction (HJ), a four-way DNA structure. At the end of recombination, HJs are cleaved by specific nucleases called resolvases. In Gram-negative bacteria, this cleavage is performed by RuvC, a dimeric endonuclease that belongs to the retroviral integrase superfamily. Here, we report the first crystal structure of RuvC in complex with a synthetic HJ solved at 3.75 Å resolution. The junction in the complex is in an unfolded 2-fold symmetrical conformation, in which the four arms point toward the vertices of a tetrahedron. The two scissile phosphates are located one nucleotide from the strand exchange point, and RuvC approaches them from the minor groove side. The key protein-DNA contacts observed in the structure were verified using a thiol-based site-specific cross-linking approach. Compared with known complex structures of the phage resolvases endonuclease I and endonuclease VII, the RuvC structure exhibits striking differences in the mode of substrate binding and location of the cleavage site.