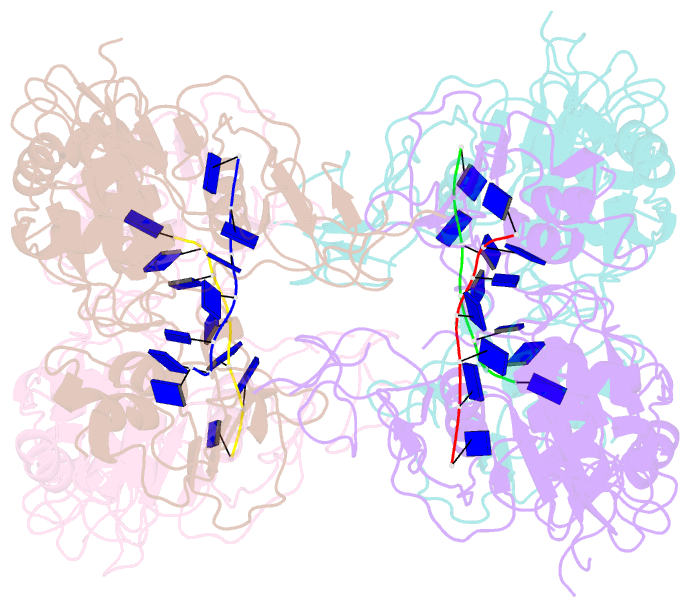
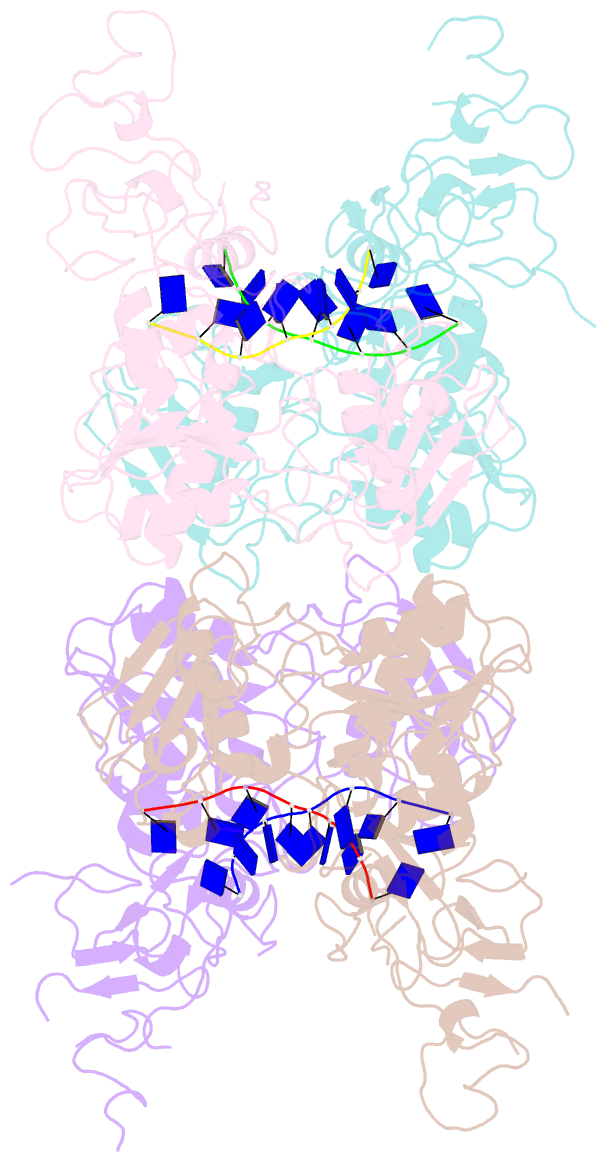Summary information and primary citation
- PDB-id
- 4n0o; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.65 Å)
- Summary
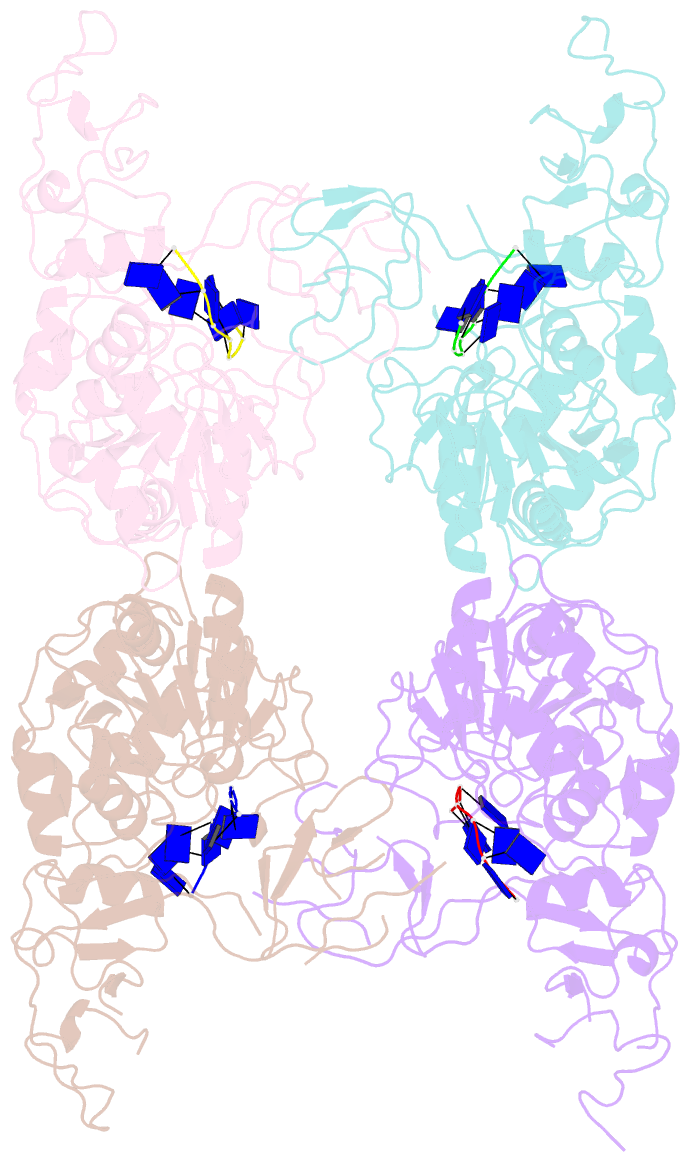
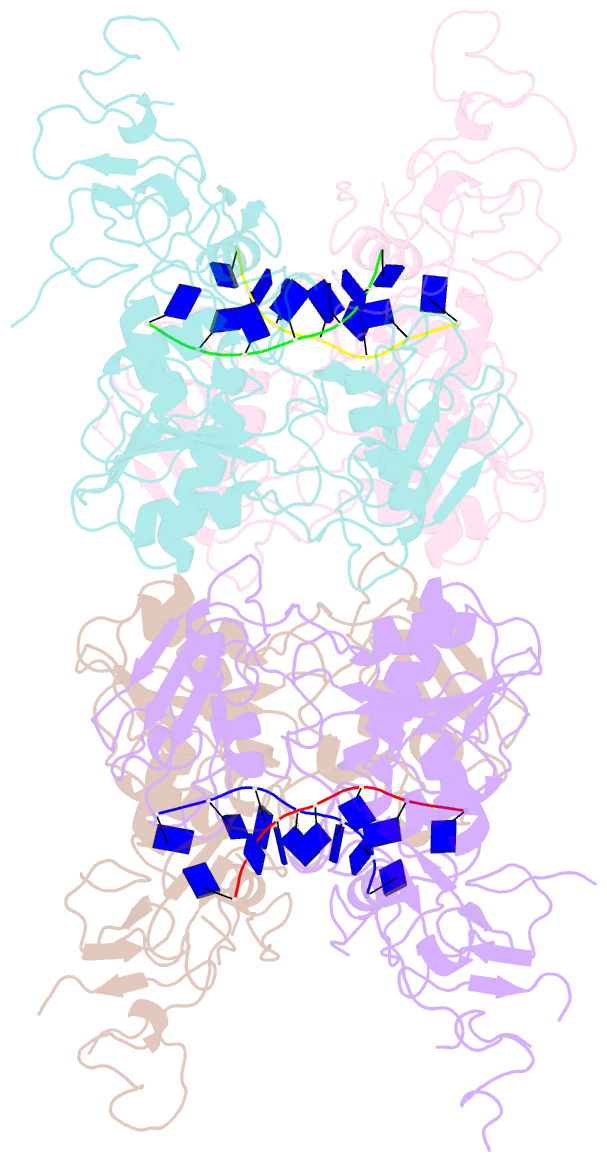
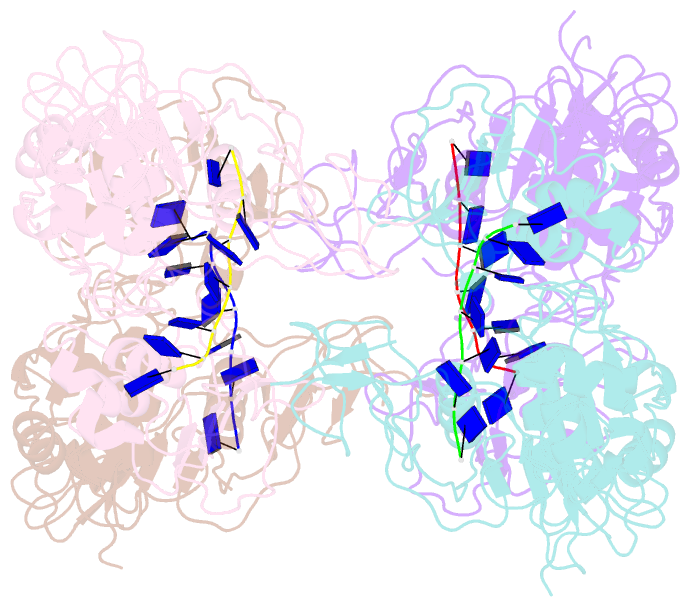
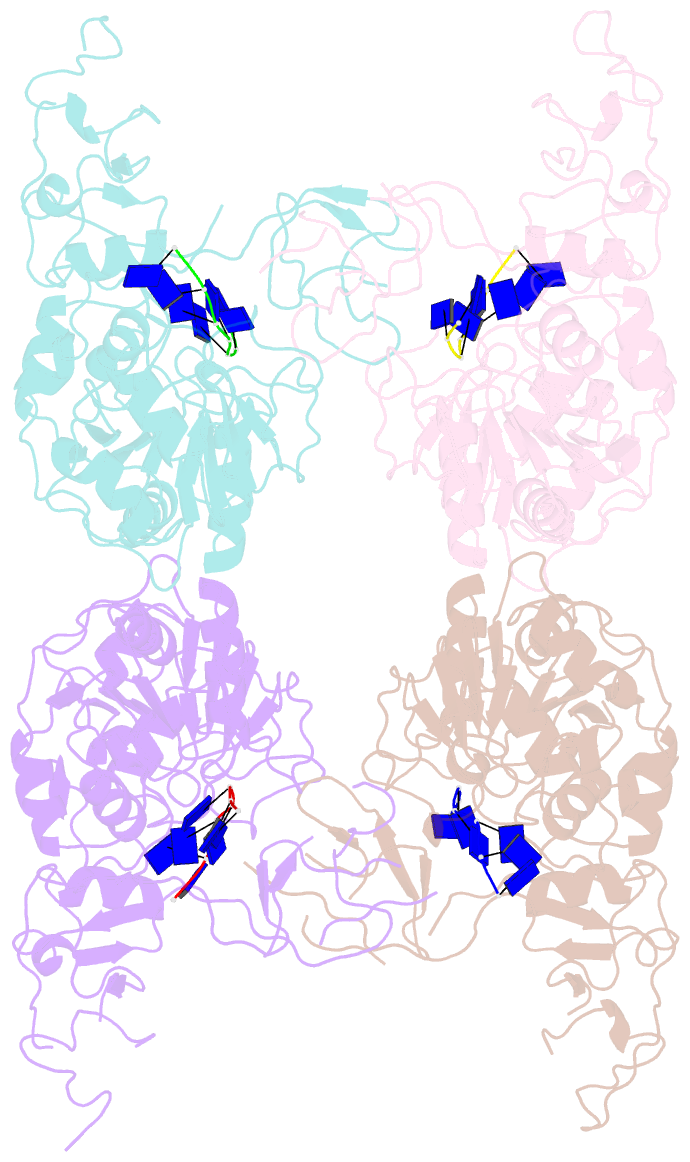
- Complex structure of arterivirus nonstructural protein 10 (helicase) with DNA
- Reference
- Deng Z, Lehmann KC, Li X, Feng C, Wang G, Zhang Q, Qi X, Yu L, Zhang X, Feng W, Wu W, Gong P, Tao Y, Posthuma CC, Snijder EJ, Gorbalenya AE, Chen Z (2014): "Structural basis for the regulatory function of a complex zinc-binding domain in a replicative arterivirus helicase resembling a nonsense-mediated mRNA decay helicase." Nucleic Acids Res., 42, 3464-3477. doi: 10.1093/nar/gkt1310.
- Abstract
- All positive-stranded RNA viruses with genomes>∼7 kb encode helicases, which generally are poorly characterized. The core of the nidovirus superfamily 1 helicase (HEL1) is associated with a unique N-terminal zinc-binding domain (ZBD) that was previously implicated in helicase regulation, genome replication and subgenomic mRNA synthesis. The high-resolution structure of the arterivirus helicase (nsp10), alone and in complex with a polynucleotide substrate, now provides first insights into the structural basis for nidovirus helicase function. A previously uncharacterized domain 1B connects HEL1 domains 1A and 2A to a long linker of ZBD, which further consists of a novel RING-like module and treble-clef zinc finger, together coordinating three Zn atoms. On substrate binding, major conformational changes were evident outside the HEL1 domains, notably in domain 1B. Structural characterization, mutagenesis and biochemistry revealed that helicase activity depends on the extensive relay of interactions between the ZBD and HEL1 domains. The arterivirus helicase structurally resembles the cellular Upf1 helicase, suggesting that nidoviruses may also use their helicases for post-transcriptional quality control of their large RNA genomes.