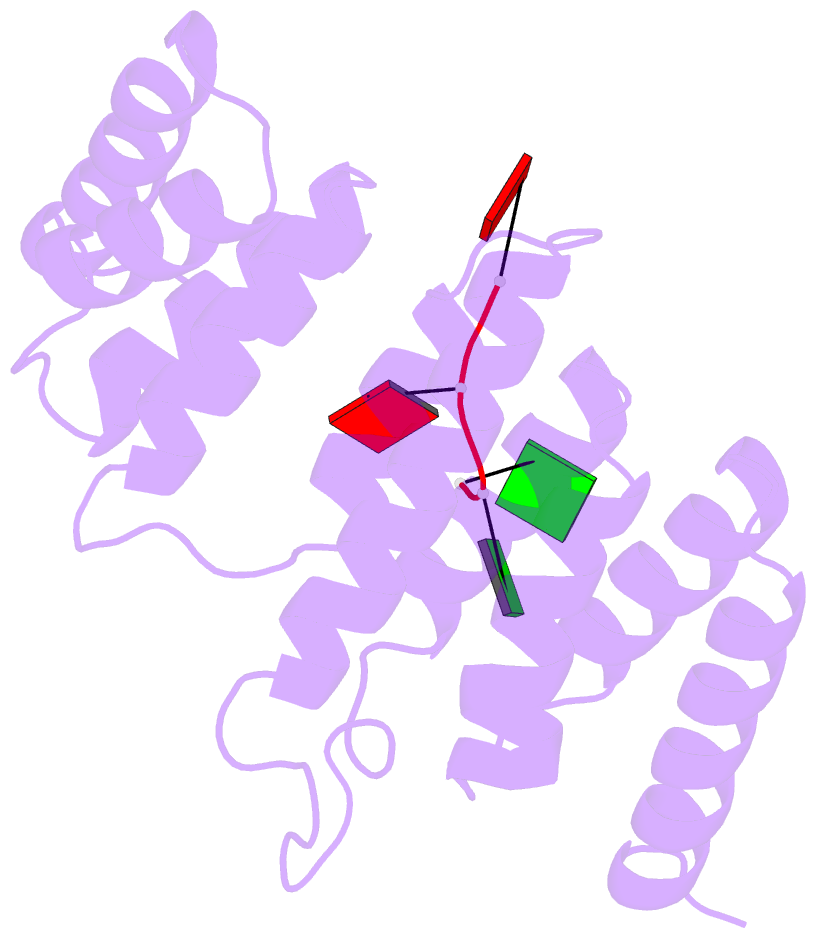
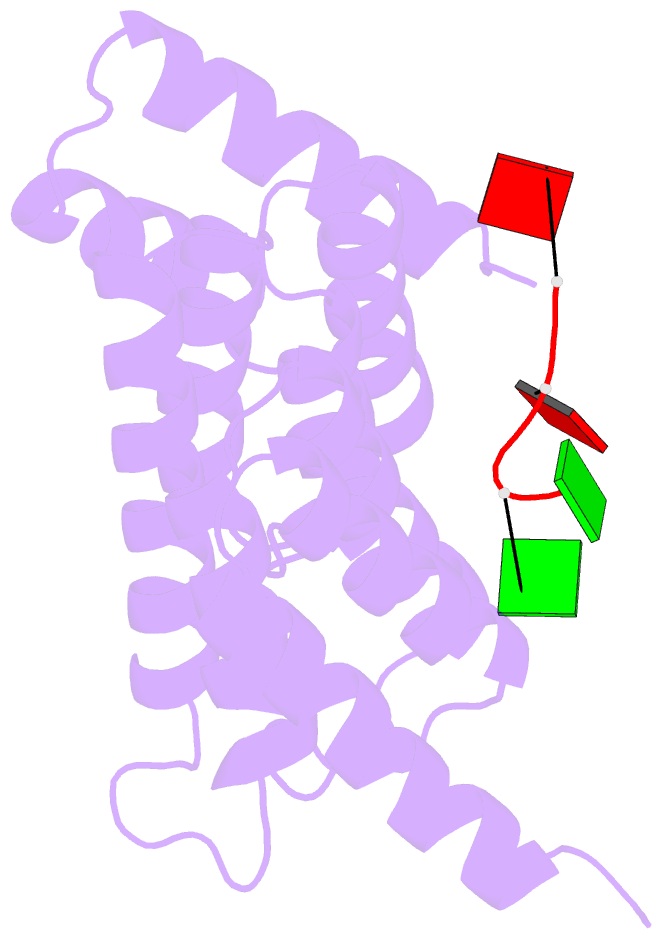
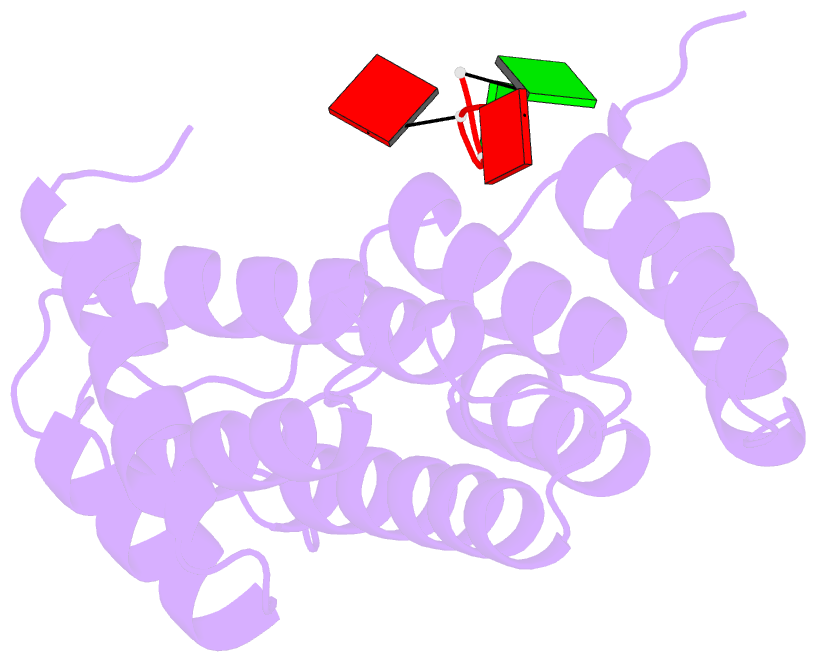
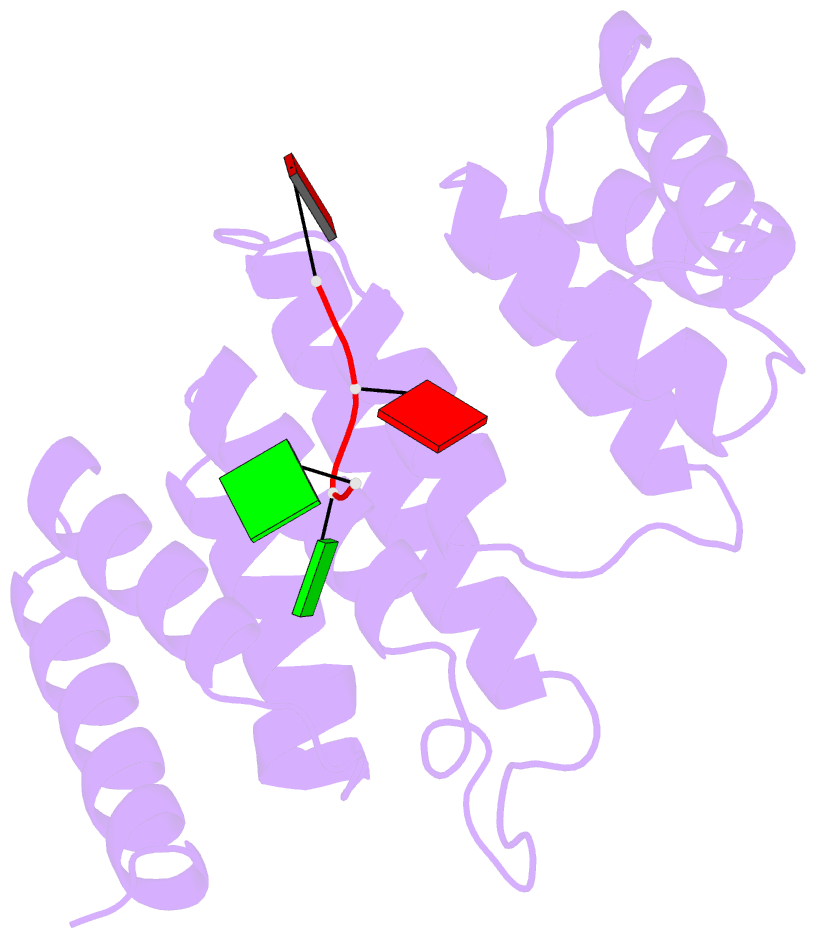
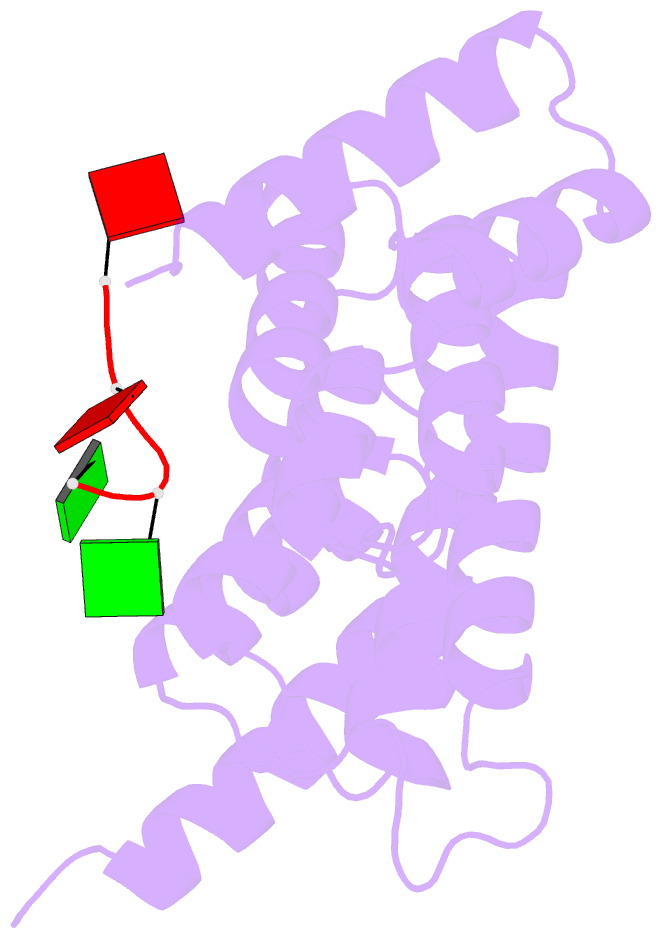
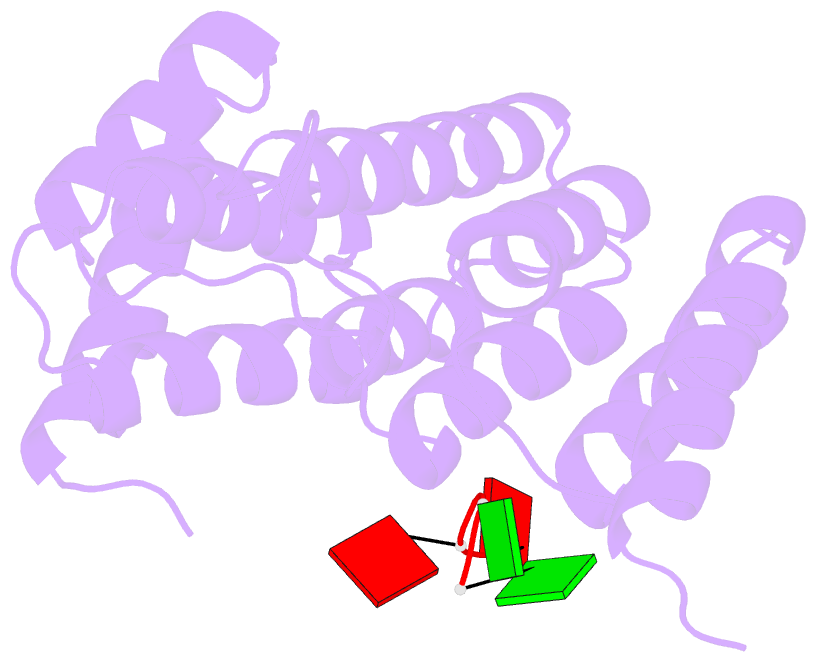
Summary information and primary citation
- PDB-id
-
4n2s;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- splicing-RNA
- Method
- X-ray (3.0 Å)
- Summary
- Crystal structure of tha8 in complex with zm1a-6
RNA
- Reference
-
Ke J, Chen RZ, Ban T, Zhou XE, Gu X, Tan MH, Chen C, Kang
Y, Brunzelle JS, Zhu JK, Melcher K, Xu HE (2013):
"Structural
basis for RNA recognition by a dimeric PPR-protein
complex." Nat.Struct.Mol.Biol.,
20, 1377-1382. doi: 10.1038/nsmb.2710.
- Abstract
- Thylakoid assembly 8 (THA8) is a pentatricopeptide
repeat (PPR) RNA-binding protein required for the splicing
of the transcript of ycf3, a gene involved in chloroplast
thylakoid-membrane biogenesis. Here we report the
identification of multiple THA8-binding sites in the ycf3
intron and present crystal structures of Brachypodium
distachyon THA8 either free of RNA or bound to two of the
identified RNA sites. The apostructure reveals a THA8
monomer with five tandem PPR repeats arranged in a planar
fold. The complexes of THA8 bound to the two short RNA
fragments surprisingly reveal asymmetric THA8 dimers with
the bound RNAs at the dimeric interface. RNA binding
induces THA8 dimerization, with a conserved G nucleotide of
the bound RNAs making extensive contacts with both
monomers. Together, these results establish a new model of
RNA recognition by RNA-induced formation of an asymmetric
dimer of a PPR protein.